-
SIED Moderators: Genetic Freak | Serotonin101
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
I like to draw random molcecules steroid discussion edition
- Thread starter black53
- Start date
I was just thinking about this earlier... or drol without methylation
I guess it would stop being orally active.oxandrolone without the 17-alpha alkylation
Anyone know if this was ever tested / found to be any good?
nolys
Bluelight Crew
Wouldn't survive the first pass through the liver
^^well obviously it'd be used as an injectable similar to the master on - superdrol relation. Yet methylation changes the effects pretty significantly so it's safe to say we'd have some very unique compounds if we removed the methyl groups on a bunch of orals. Also why no addition of esters to methylated compounds? Or is it not chemically possible? Haven't taken organic chem yet so still learning.
If you mean why no esters on my pics - it's because I couldn't be bothered to draw them, right now it's demethylated oxandrolone no ester and demethylated oxymetholone no ester, but you could add whichever you wanted. If you mean why don't methylated compounds have esters, the ester goes in place of the methyl group.
nolys, flyhighk, yes these would be injectable compounds, still wonder about their effect, like Serotonin101 said they could be completely different (think dbol and boldenone as another example)... good? bad? average?....
and the dimethyltren should be beastly if it's close to dimethylnandrolone (your liver would hate you
nolys, flyhighk, yes these would be injectable compounds, still wonder about their effect, like Serotonin101 said they could be completely different (think dbol and boldenone as another example)... good? bad? average?....
and the dimethyltren should be beastly if it's close to dimethylnandrolone (your liver would hate you
^^I think I've read of a dimethyltrienolone as a designer steroid. And thanks for the ester clarification. I was thinking of the eq-dbol relationship as well. whyarewenotfundingthis.jpg
I think most of the pharm companies have switched over to SARMS due to the stigma attached to anabolic steroids, but I'm sure underground labs making stuff for high level athletes are still producing/researching new and exciting compounds. Unfortunately they can't really publish their results....
-Guido-
Bluelight Crew
- Joined
- Nov 1, 2004
- Messages
- 34,834
^^I think I've read of a dimethyltrienolone as a designer steroid. And thanks for the ester clarification. I was thinking of the eq-dbol relationship as well. whyarewenotfundingthis.jpg
Yes, Dimthyltrienolone was produced by pharmaceutical companies in the past but research was scrapped due to the fact it destroyed the livers of lab animals so terribly there was no reason to even try human studies.
No UGL produces this due to the fact it can't be used effectively for bodybuilding because of the hepatoxicity it carries.
-Guido-
Bluelight Crew
- Joined
- Nov 1, 2004
- Messages
- 34,834
I'd like to see the nonmethyl version of oxandrolone made. I wonder how much the properties would change. If we're lucky and they don't change much, you'd have a great compound.
What bothers me sometimes, is that there is probably a few guys out there cooking up stuff like that and they are experimenting on themselves or a few lucky clients.
.........Ok, first, thank you both for the reply.
Yeah, found quite a lot about MENT, so no more questions about that. And what he said is about the same as I found on my own.
Is he sure that dimethyl tren and mibolerone are the same thing?
This is tren:
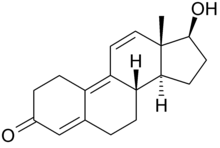
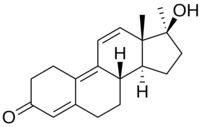
This is methyltren:
This is mibolerone:
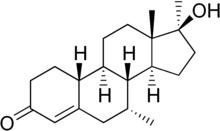
Isn't mibolerone missing some two double bonds to be dimethyl tren?
As to why the 7a methyl does what it does good question.
If I'm reading this correctly 7a-methyltren hasn't been tested as far as he is aware, right?
And regarding the oxandrolone without the 17aa anything about that?
......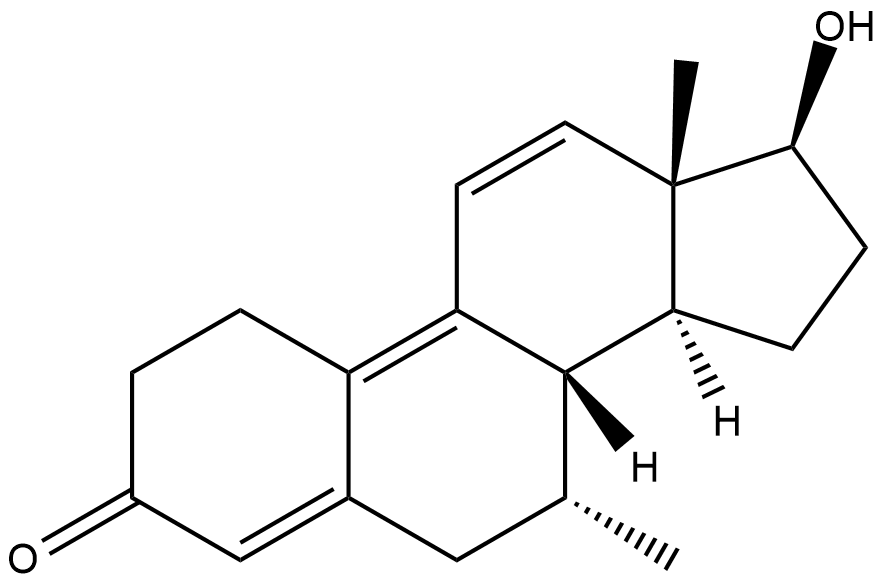
7a-methyltren, what I'd like to see tested
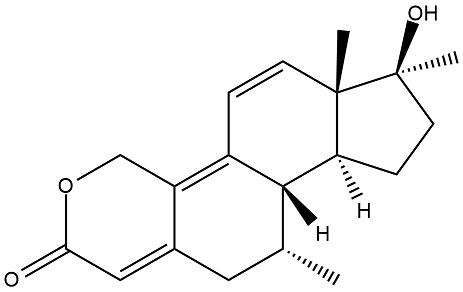
Some kind of combination of dimethyltren and oxandrolone. If it's too toxic remove the 17a methyl
Some posts are missing imo, I'll add copies of the important parts (or the ones I remember).
Why 7a-methyl tren?
A bit of extrapolation of the effects of the 7a methyl in other compounds (think I already posted about the dbol modified in the same way) - usually stronger compounds, but not as toxic as the 17a methyl or 7a 17a dimethyl ones. Call it a gut feeling if you will. I know it wouldn't be enough to convince anyone to make it, but if I were rich I'd pay for the synth (and many others), just to see how the new compounds would compare. Could be toxic crap, could be meh, could be the next big thing.
I'd also be interested in exploring oxandrolone modifications. And try making one of the better AAS even better.
Why the 7a,17a oxandrotren?
Wouldn't really be a combination of oxandrolone and tren without it. And to hopefully bring along some of oxandrolones good effects. The basic tren structure because we know this works. The 7a methyl because it improves the potency of many compounds. And the 17a methyl because it makes them even stronger. What this would actually do, I have no idea... just looks interesting to me.
Anyone else have some stuff they'd like to see made?
Big Cat Reply:
Isn't mibolerone missing some two double bonds to be dimethyl tren?
You are correct. Guess I've had that wrong in my head for quite some time now. Mibolerone would be 17a-MENT.
Considering the toxicity of this (mcg dose sublingual) and methyltren (1-2mg day inject), I'm not sure you'd actually want to test dimethyltren even acutely.
Quote
If I'm reading this correctly 7a-methyltren hasn't been tested as far as you are aware, right..?
At least not in any studies I've read.
Quote
And regarding the oxandrolone without the 17aa anything about that?
No actual data or experience no, but as a general rule of thumb non-17AA versions are acutely weaker, but turn out to be solid, relatively mild compounds for long term use that are well liked and tolerated (EQ and Mast vs Dbol and SD for example), because of the ability to maintain steady levels for longer times. However permit me to make the analogy with 17AA-less stanozolol, here you'd be starting with a notably weak compound, unlikely to add significant mass, so the result is likely to be dissapointing unless it has some sort of feelgood effect or something.
Isn't mibolerone missing some two double bonds to be dimethyl tren?
You are correct. Guess I've had that wrong in my head for quite some time now. Mibolerone would be 17a-MENT.
Considering the toxicity of this (mcg dose sublingual) and methyltren (1-2mg day inject), I'm not sure you'd actually want to test dimethyltren even acutely.
Quote
If I'm reading this correctly 7a-methyltren hasn't been tested as far as you are aware, right..?
At least not in any studies I've read.
Quote
And regarding the oxandrolone without the 17aa anything about that?
No actual data or experience no, but as a general rule of thumb non-17AA versions are acutely weaker, but turn out to be solid, relatively mild compounds for long term use that are well liked and tolerated (EQ and Mast vs Dbol and SD for example), because of the ability to maintain steady levels for longer times. However permit me to make the analogy with 17AA-less stanozolol, here you'd be starting with a notably weak compound, unlikely to add significant mass, so the result is likely to be dissapointing unless it has some sort of feelgood effect or something.
Thanks for the reply (and for copying them around).
I guess well just have to wait until someone makes 7a-methyl tren and see what happens with the effects/side effects. Same for 7a 17a oxandrotren and the other stuff I drew. I'm sure others have had similar ideas
As for the non 17aa oxandrolone... yeah, if you're looking for size it isn't for you. But I like it's effects on strength, fat, lack of side effects... So if it was just a weaker version that would need to be dosed higher people would still be interested (well those who could afford it anyway). And it may be a great compound for women.
So, anyone making new steroids and reading this - 7a methyl tren, 7a 17a oxandrolone, 7a oxandrolone without the 17a methyl and http://i.imgur.com/DyagECS.png without the 17a methyl please

I guess well just have to wait until someone makes 7a-methyl tren and see what happens with the effects/side effects. Same for 7a 17a oxandrotren and the other stuff I drew. I'm sure others have had similar ideas
As for the non 17aa oxandrolone... yeah, if you're looking for size it isn't for you. But I like it's effects on strength, fat, lack of side effects... So if it was just a weaker version that would need to be dosed higher people would still be interested (well those who could afford it anyway). And it may be a great compound for women.
So, anyone making new steroids and reading this - 7a methyl tren, 7a 17a oxandrolone, 7a oxandrolone without the 17a methyl and http://i.imgur.com/DyagECS.png without the 17a methyl please

Last edited by a moderator:
.............The first 'designer steroid' was THG
which was discovered by Patrick Arnold at BALCO. Synthesis is via hydrogenation of gestinone.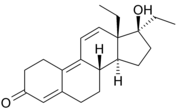
Only a few years later, The Greek olympic weightlifting team were caught using R1881
You can see how similar they are (1 methyl).
I'm not well up on the situation with anabolic steroids, but are people still producing newer and newer analogues?









