Transform
Bluelight Crew
- Joined
- Sep 5, 2010
- Messages
- 4,807
In doing the research for the classification of these drugs, the UK ACMD has apparently commissioned research into the binding profiles. It's a shame that this ban will blanket a huge range of compounds but at least something positive has come from it. The full report can be found here (p14) and is incredibly interesting as a review of what's known about the arylcyclohexamines.
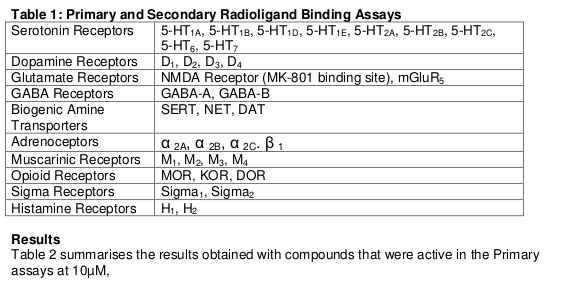 Table 2: Results of Binding assays – pKi , (Ki) nM
Table 2: Results of Binding assays – pKi , (Ki) nM
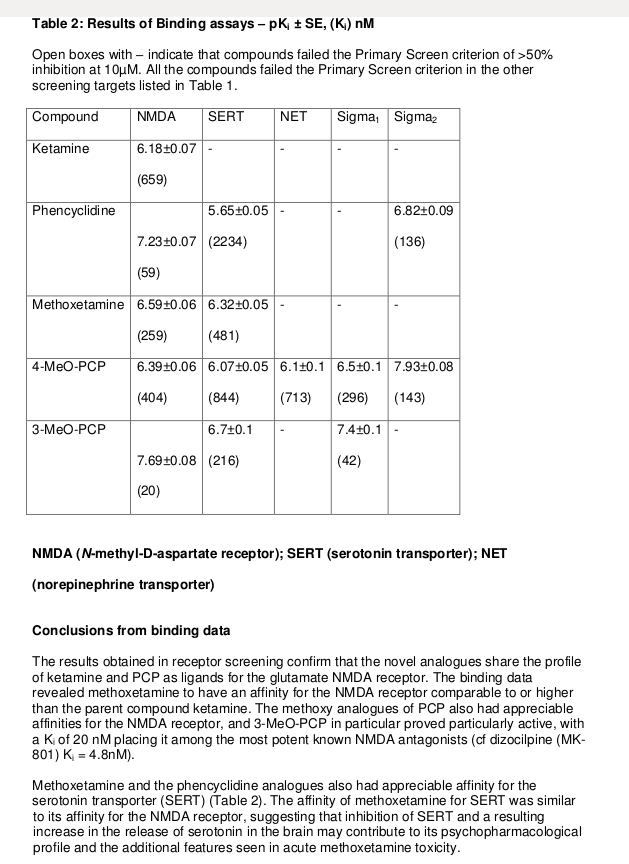
Open boxes with – indicate that compounds failed the Primary Screen criterion of >50% inhibition at 10μM. All the compounds failed the Primary Screen criterion in the other screening targets listed in Table 1.
[table="width: 500, class: grid"]
[tr]
[td]Compound[/td]
[td]NMDA [/td]
[td]SERT[/td]
[td]NET[/td]
[td]Sigma1[/td]
[td]Sigma2[/td]
[/tr]
[tr]
[td]Ketamine[/td]
[td]6.18 (659)[/td]
[td]-[/td]
[td]-[/td]
[td]-[/td]
[td]-[/td]
[/tr]
[tr]
[td]Phencyclidine[/td]
[td]7.23 (59)[/td]
[td]5.65 (2234)[/td]
[td]-[/td]
[td]-[/td]
[td]6.82 (136)[/td]
[/tr]
[tr]
[td]MXE[/td]
[td]6.59 (259)[/td]
[td]6.32 (481)[/td]
[td]-[/td]
[td]-[/td]
[td]-[/td]
[/tr]
[tr]
[td]4-MeO-PCP[/td]
[td]6.39 (404)[/td]
[td]6.07 (844)[/td]
[td]6.1 (713)[/td]
[td]6.5 (296)[/td]
[td]7.93 (143)[/td]
[/tr]
[tr]
[td]3-MeO-PCP[/td]
[td]7.69 (20)[/td]
[td]6.7 (216)[/td]
[td]-[/td]
[td]7.4 (42)[/td]
[td]-[/td]
[/tr]
[/table]
Conclusions from binding data
The results obtained in receptor screening confirm that the novel analogues share the profile of ketamine and PCP as ligands for the glutamate NMDA receptor. The binding data revealed methoxetamine to have an affinity for the NMDA receptor comparable to or higher than the parent compound ketamine. The methoxy analogues of PCP also had appreciable affinities for the NMDA receptor, and 3-MeO-PCP in particular proved particularly active, with a Ki of 20 nM placing it among the most potent known NMDA antagonists (cf dizocilpine (MK-801) Ki = 4.8nM).
Methoxetamine and the phencyclidine analogues also had appreciable affinity for the serotonin transporter (SERT) (Table 2). The affinity of methoxetamine for SERT was similar to its affinity for the NMDA receptor, suggesting that inhibition of SERT and a resulting increase in the release of serotonin in the brain may contribute to its psychopharmacological profile and the additional features seen in acute methoxetamine toxicity.
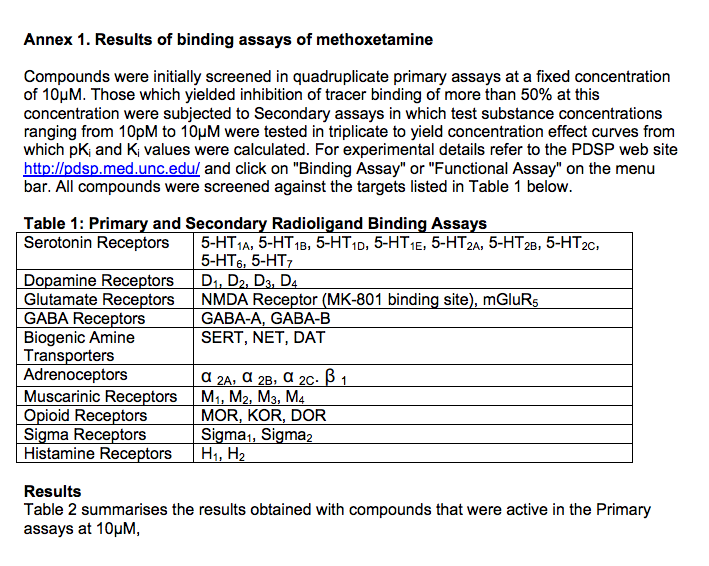
Compounds were initially screened in quadruplicate primary assays at a fixed concentration of 10μM. Those which yielded inhibition of tracer binding of more than 50% at this concentration were subjected to Secondary assays in which test substance concentrations ranging from 10pM to 10μM were tested in triplicate to yield concentration effect curves from which pKi and Ki values were calculated.
For experimental details refer to the PDSP web site http://pdsp.med.unc.edu/ and click on "Binding Assay" or "Functional Assay" on the menu bar. All compounds were screened against the targets listed in Table 1 below.

Open boxes with – indicate that compounds failed the Primary Screen criterion of >50% inhibition at 10μM. All the compounds failed the Primary Screen criterion in the other screening targets listed in Table 1.
[table="width: 500, class: grid"]
[tr]
[td]Compound[/td]
[td]NMDA [/td]
[td]SERT[/td]
[td]NET[/td]
[td]Sigma1[/td]
[td]Sigma2[/td]
[/tr]
[tr]
[td]Ketamine[/td]
[td]6.18 (659)[/td]
[td]-[/td]
[td]-[/td]
[td]-[/td]
[td]-[/td]
[/tr]
[tr]
[td]Phencyclidine[/td]
[td]7.23 (59)[/td]
[td]5.65 (2234)[/td]
[td]-[/td]
[td]-[/td]
[td]6.82 (136)[/td]
[/tr]
[tr]
[td]MXE[/td]
[td]6.59 (259)[/td]
[td]6.32 (481)[/td]
[td]-[/td]
[td]-[/td]
[td]-[/td]
[/tr]
[tr]
[td]4-MeO-PCP[/td]
[td]6.39 (404)[/td]
[td]6.07 (844)[/td]
[td]6.1 (713)[/td]
[td]6.5 (296)[/td]
[td]7.93 (143)[/td]
[/tr]
[tr]
[td]3-MeO-PCP[/td]
[td]7.69 (20)[/td]
[td]6.7 (216)[/td]
[td]-[/td]
[td]7.4 (42)[/td]
[td]-[/td]
[/tr]
[/table]
Conclusions from binding data
The results obtained in receptor screening confirm that the novel analogues share the profile of ketamine and PCP as ligands for the glutamate NMDA receptor. The binding data revealed methoxetamine to have an affinity for the NMDA receptor comparable to or higher than the parent compound ketamine. The methoxy analogues of PCP also had appreciable affinities for the NMDA receptor, and 3-MeO-PCP in particular proved particularly active, with a Ki of 20 nM placing it among the most potent known NMDA antagonists (cf dizocilpine (MK-801) Ki = 4.8nM).
Methoxetamine and the phencyclidine analogues also had appreciable affinity for the serotonin transporter (SERT) (Table 2). The affinity of methoxetamine for SERT was similar to its affinity for the NMDA receptor, suggesting that inhibition of SERT and a resulting increase in the release of serotonin in the brain may contribute to its psychopharmacological profile and the additional features seen in acute methoxetamine toxicity.

Last edited: