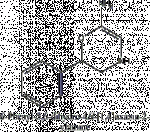Reminisant B
Greenlighter
- Joined
- Nov 3, 2005
- Messages
- 401
Just browsing wikipedia and came across a drug called radaxafine which is apparatenly a potent metabolite of bupropion/wellbutrin/zyban.
Turns out its a substituted phenmetrazine.
Do you think the same metabolism may occur in say Ethylone? (as shown in the diagrams below)
Turns out its a substituted phenmetrazine.
Do you think the same metabolism may occur in say Ethylone? (as shown in the diagrams below)