honeywhite
Bluelighter
- Joined
- Apr 5, 2012
- Messages
- 90
A few days ago, I went to a garden party. I was fortunate enough to become acquainted with a very nice and very knowledgeable physician of German extraction. This gentleman was into retirement age but still in practice as it is his passion. As the hours ticked by, the conversation naturally flowed to one specific opiate, and I learned some interesting history. I'll put it out for you here, plus fill in some gaps that I researched out of pure boredom.
Dihydromorphinone, or hydromorphone, was discovered by a company called Knoll. It was, until relatively recently, a subsidiary of BASF, which is itself a quasi-subsidiary of Interessen-Gemeinschaft Farbenindustrie, aka IG Farben (which technically isn't a corporation in the English sense of the word---closer to a price-coordinating cartel). IG Farben, at last count, had well over half a million employees. Anyway, Knoll was sold to Abbott, which is a drug manufacturer (IG makes mostly dyes, photographic film, magnetic tape, the teflon that shopping bags are made of---which is why teflon is called Igelit in German---Zyklon B and Uragan D2 HCN fumigants---it's the German equivalent to DuPont). What's this got to do with anything? Fuck all---I'm just including it here for completeness.
EDIT: The rights to make Dilaudid were recently sold to Purdue Pharma, the kind folks who brought you OxyContin, CodeineContin, HydromorphContin, and MSContin. The new imprint is a capital P; the formulation is the same as ever.

Anyway, before 1970-ish (right around the time medicine started being sold in metric units), wet Dilaudid ampoules for injection were rare. As in hen's teeth rare; they were perishable and mostly sold to hospitals and the like. For field-expedient use, in first-aid kits and the like, you'd usually find a glass tube of Dilaudid Hypodermic Tablets. You would use them as follows: you'd empty a 1/2 dram ampoule of Water for Injection BP into a clean container. You'd then drop in as many Dilaudid Hypodermic Tablets as needed (1/32 grain, 1/16 grain, and 1/8 grain strengths) into the container, and stir with a glass stirring rod. You'd then inject the medicine as usual.
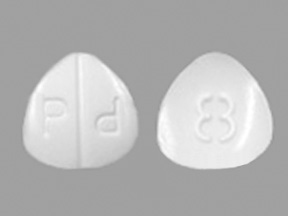
When the production of wet ampoules became more common, hypodermic tablets went out of production. Except not really. The packaging was changed to remove the word "hypodermic", but the ingredients and production method remained the same. If you get Knoll Dilaudid on prescription, identifiable either by the letter K, P, or lowercase letter a on one side, you are getting LITERALLY the same tablets as used to be used BY DOCTORS for injection. Therefore, minimal harm is engendered by using the tablets for their original intended use.
One more opiate is sold in this fashion. In the UK, it's sold under a plethora of brand names. Here are just a few: Diacephin, Diagesil, Diaphin, Diaphorm, and Morphacetin. Bayer also produces it as Heroin; there are 10 and 30 mg dry ampoules (to be mixed with Water for Injection as detailed above), and 10 mg no-longer-advertised-as-hypodermic tablets (ditto). Morphacetin et al. have largely displaced the other salts of morphine (sulphate, tartrate, phosphate, hydrochloride) in clinical use, especially in paediatric and oncological use.
Dihydromorphinone, or hydromorphone, was discovered by a company called Knoll. It was, until relatively recently, a subsidiary of BASF, which is itself a quasi-subsidiary of Interessen-Gemeinschaft Farbenindustrie, aka IG Farben (which technically isn't a corporation in the English sense of the word---closer to a price-coordinating cartel). IG Farben, at last count, had well over half a million employees. Anyway, Knoll was sold to Abbott, which is a drug manufacturer (IG makes mostly dyes, photographic film, magnetic tape, the teflon that shopping bags are made of---which is why teflon is called Igelit in German---Zyklon B and Uragan D2 HCN fumigants---it's the German equivalent to DuPont). What's this got to do with anything? Fuck all---I'm just including it here for completeness.
EDIT: The rights to make Dilaudid were recently sold to Purdue Pharma, the kind folks who brought you OxyContin, CodeineContin, HydromorphContin, and MSContin. The new imprint is a capital P; the formulation is the same as ever.

Anyway, before 1970-ish (right around the time medicine started being sold in metric units), wet Dilaudid ampoules for injection were rare. As in hen's teeth rare; they were perishable and mostly sold to hospitals and the like. For field-expedient use, in first-aid kits and the like, you'd usually find a glass tube of Dilaudid Hypodermic Tablets. You would use them as follows: you'd empty a 1/2 dram ampoule of Water for Injection BP into a clean container. You'd then drop in as many Dilaudid Hypodermic Tablets as needed (1/32 grain, 1/16 grain, and 1/8 grain strengths) into the container, and stir with a glass stirring rod. You'd then inject the medicine as usual.
When the production of wet ampoules became more common, hypodermic tablets went out of production. Except not really. The packaging was changed to remove the word "hypodermic", but the ingredients and production method remained the same. If you get Knoll Dilaudid on prescription, identifiable either by the letter K, P, or lowercase letter a on one side, you are getting LITERALLY the same tablets as used to be used BY DOCTORS for injection. Therefore, minimal harm is engendered by using the tablets for their original intended use.
One more opiate is sold in this fashion. In the UK, it's sold under a plethora of brand names. Here are just a few: Diacephin, Diagesil, Diaphin, Diaphorm, and Morphacetin. Bayer also produces it as Heroin; there are 10 and 30 mg dry ampoules (to be mixed with Water for Injection as detailed above), and 10 mg no-longer-advertised-as-hypodermic tablets (ditto). Morphacetin et al. have largely displaced the other salts of morphine (sulphate, tartrate, phosphate, hydrochloride) in clinical use, especially in paediatric and oncological use.
Last edited: