moracca
Bluelighter
- Joined
- Jul 16, 2005
- Messages
- 261
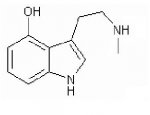
Ok, so after looking through most of the clever RC tryptamine variations, one thing stands out to me. All of these compounds have 2 seperate chains attached to the nitrogen atom. (for example, 4-hydroxy,DImethyl-T, or 4-ho-DIisopropyltryptamine) My question is this, why can i find no information about compounds that have only one carbon chain coming off the N, with the other chain replaced by a hydrogen atom. for example, 4-hydroxy,N,N-methyl-T (shown in attatched image), or 4-aco,N,N-isopropyl-T. Is there some reason that this sort of compound would be inactive? I just haven't been able to find anything about them, and would appreciate those of you with a bit more knowledge on the subject to chime in and let me know the error of my logic.
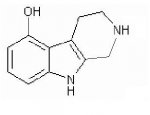
Also... the second image is another offshoot idea I had while thinking about this question... I kind of doubt that it would be possible, or active, but then again, why not? any help is appreciated. Thanks!
Also... the second image is another offshoot idea I had while thinking about this question... I kind of doubt that it would be possible, or active, but then again, why not? any help is appreciated. Thanks!