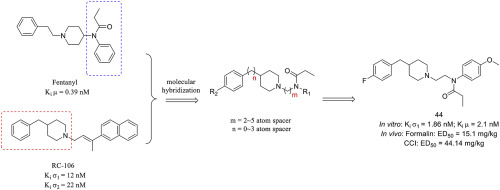
TheBlackPirate
Bluelighter
- Joined
- Dec 16, 2015
- Messages
- 680
Int J Neuropsychopharmacol. 2019 Sep; 22(9): 601–607.
Published online 2019 Jul 29. doi: 10.1093/ijnp/pyz042
PMCID: PMC6754733
PMID: 31353393
Opportunities in Novel Psychotropic Drug Design from Natural Compounds
Abstract
Multiple initiatives at the national and international level support natural drug discovery. Psychiatrists and patients are not well informed about natural psychotropics in general. Existing antidepressant and antipsychotic drugs were developed from atropine, a natural product. Subsequent drug developments were largely based on extension and modification of earlier molecular scaffolds. This limits their mechanisms of action to similar neuropathways. Natural psychotropic substances, particularly those with hallucinogenic and psychedelic properties and different chemical structures, may serve as new paths to novel psychotropic drug development.
Keywords: natural psychoactive substances, botanical psychotropics, designer drugs, psychotropic designs
Introduction
Natural psychotropic research has not been at the forefront of psychopharmacology until recently. In 2019, the World Health Organization will include traditional medicine (TM) into its global compendium. The US National Cancer Institute (NCI) announced a new initiative for drug discovery from natural products (Thornburg et al., 2018). The NCI’s natural product repository consists of over 230 000 natural product extracts collected from biodiverse areas in different parts of the world. The US National Center for Complementary and Integrative Health of the National Institutes of Health announced a similar program on natural product drug discovery (NCCIH, 2016). All of these initiatives have generated much interest and discussion regarding medicines and psychotropics derived from plants, animals, and minerals.
The recent increase in interest regarding natural psychotropics has been fueled by the emerging threat of new psychoactive substances worldwide, as alerted by the United Nations Office on Drugs and Crime (UNODC, 2019). New psychoactive substances are mostly available as natural products, such as cacti and animal venom or from modification of their active ingredients, such as synthetic cannabinoids.
Health supplements containing TM are a big business. The absence of strict regulation and the adulteration of some of these products has triggered repeated warnings from the US Food and Drug Administration (FDA, 2019a) and the implementation of stricter oversight this year (FDA, 2019b). Yet based on the sales figures of these supplements, it is clear that TM is highly popular. One of the reasons why patients have resorted to TM may be related to the belief that TM products are “natural” and therefore “safe.” As a result, patient concerns about the safety of manmade drugs may lead to noncompliance, especially during long-term drug maintenance. A significant number of patients suffering from mood disorders failed to respond to existing antidepressant drugs according to large-scale studies such as Star* D (Trivedi et al., 2006). Desperate patients understandably might resort to other forms of medicine that claim efficacy. The success of TM has shown that there is an unmet need for new psychiatric drugs to serve many patients who are not satisfied with current psychiatric drugs. The US National Institute of Mental Health psychoactive drug screening program (NIMH, 2017) represents an effort to stimulate innovative research to discover novel tools and potential therapeutic agents for the treatment of psychiatric disorders, complementary to the NCI and National Institutes of Health initiatives.
Many patients and clinicians alike, however, are not well-informed about TM and may not know that existing antidepressant and antipsychotic drugs in fact are based on plant molecules. Later generations of antidepressant and antipsychotics were mostly synthesized from drug molecules modified from earlier scaffolds. Discovery of psychotropic molecules, natural or synthetic, has mostly been serendipitous. New molecules with targets different from the current are urgently needed. We review in this manuscript the evolution of psychiatric drugs from natural molecules, the merits of this approach, and future opportunities in designer drugs.
...
Conclusion
Natural medicine is popular, but its true value is found in its active ingredients and novel molecules. Phytochemicals like atropine have proven to be valuable scaffolds or chemical platforms from which new drugs were developed. Our existing antipsychotics and antidepressants originated from atropine through serendipity, but serendipity only occurs with the prepared mind. Many of the important drug discoveries have occurred through careful clinical observation of unexpected or unusual effects of compounds with other indications. The recent surge in neuroscience and neuropharmacology research related to new psychoactive substances may open new paths to novel compounds, with alternate mechanisms of action on psychiatric disorders. Neuropsychopharmacologists and clinicians together will continue to play critical but cooperative roles in future discoveries.
Full Medical Journal Paper: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6754733/
Published online 2019 Jul 29. doi: 10.1093/ijnp/pyz042
PMCID: PMC6754733
PMID: 31353393
Opportunities in Novel Psychotropic Drug Design from Natural Compounds
Abstract
Multiple initiatives at the national and international level support natural drug discovery. Psychiatrists and patients are not well informed about natural psychotropics in general. Existing antidepressant and antipsychotic drugs were developed from atropine, a natural product. Subsequent drug developments were largely based on extension and modification of earlier molecular scaffolds. This limits their mechanisms of action to similar neuropathways. Natural psychotropic substances, particularly those with hallucinogenic and psychedelic properties and different chemical structures, may serve as new paths to novel psychotropic drug development.
Keywords: natural psychoactive substances, botanical psychotropics, designer drugs, psychotropic designs
Introduction
Natural psychotropic research has not been at the forefront of psychopharmacology until recently. In 2019, the World Health Organization will include traditional medicine (TM) into its global compendium. The US National Cancer Institute (NCI) announced a new initiative for drug discovery from natural products (Thornburg et al., 2018). The NCI’s natural product repository consists of over 230 000 natural product extracts collected from biodiverse areas in different parts of the world. The US National Center for Complementary and Integrative Health of the National Institutes of Health announced a similar program on natural product drug discovery (NCCIH, 2016). All of these initiatives have generated much interest and discussion regarding medicines and psychotropics derived from plants, animals, and minerals.
The recent increase in interest regarding natural psychotropics has been fueled by the emerging threat of new psychoactive substances worldwide, as alerted by the United Nations Office on Drugs and Crime (UNODC, 2019). New psychoactive substances are mostly available as natural products, such as cacti and animal venom or from modification of their active ingredients, such as synthetic cannabinoids.
Health supplements containing TM are a big business. The absence of strict regulation and the adulteration of some of these products has triggered repeated warnings from the US Food and Drug Administration (FDA, 2019a) and the implementation of stricter oversight this year (FDA, 2019b). Yet based on the sales figures of these supplements, it is clear that TM is highly popular. One of the reasons why patients have resorted to TM may be related to the belief that TM products are “natural” and therefore “safe.” As a result, patient concerns about the safety of manmade drugs may lead to noncompliance, especially during long-term drug maintenance. A significant number of patients suffering from mood disorders failed to respond to existing antidepressant drugs according to large-scale studies such as Star* D (Trivedi et al., 2006). Desperate patients understandably might resort to other forms of medicine that claim efficacy. The success of TM has shown that there is an unmet need for new psychiatric drugs to serve many patients who are not satisfied with current psychiatric drugs. The US National Institute of Mental Health psychoactive drug screening program (NIMH, 2017) represents an effort to stimulate innovative research to discover novel tools and potential therapeutic agents for the treatment of psychiatric disorders, complementary to the NCI and National Institutes of Health initiatives.
Many patients and clinicians alike, however, are not well-informed about TM and may not know that existing antidepressant and antipsychotic drugs in fact are based on plant molecules. Later generations of antidepressant and antipsychotics were mostly synthesized from drug molecules modified from earlier scaffolds. Discovery of psychotropic molecules, natural or synthetic, has mostly been serendipitous. New molecules with targets different from the current are urgently needed. We review in this manuscript the evolution of psychiatric drugs from natural molecules, the merits of this approach, and future opportunities in designer drugs.
...
Conclusion
Natural medicine is popular, but its true value is found in its active ingredients and novel molecules. Phytochemicals like atropine have proven to be valuable scaffolds or chemical platforms from which new drugs were developed. Our existing antipsychotics and antidepressants originated from atropine through serendipity, but serendipity only occurs with the prepared mind. Many of the important drug discoveries have occurred through careful clinical observation of unexpected or unusual effects of compounds with other indications. The recent surge in neuroscience and neuropharmacology research related to new psychoactive substances may open new paths to novel compounds, with alternate mechanisms of action on psychiatric disorders. Neuropsychopharmacologists and clinicians together will continue to play critical but cooperative roles in future discoveries.
Full Medical Journal Paper: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6754733/