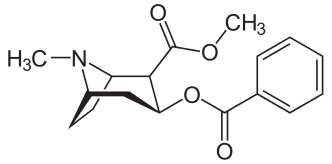
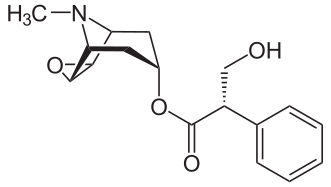
L-Methylphenidate Is Stereoselectively Hydrolyzed by Human Carboxylesterase CES1A1
Zejin Sun, Daryl J. Murry, Sonal P. Sanghani, Wilhelmina I. Davis, Natalia Y. Kedishvili, Qin Zou, Thomas D. Hurley and William F. Bosron
http://jpet.aspetjournals.org/content/310/2/469.long
Clinically relevant doses of methylphenidate significantly occupy norepinephrine transporters in humans in vivo.
Hannestad J, Gallezot JD, Planeta-Wilson B, Lin SF, Williams WA, van Dyck CH, Malison RT, Carson RE, Ding YS.
http://www.ncbi.nlm.nih.gov/pubmed/20691429
Medical Marijuana: Clearing Away the Smoke
Igor Grant, J. Hampton Atkinson, Ben Gouaux, and Barth Wilsey
http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3358713
As with all medications, benefits and risks need to be weighed in recommending cannabis to patients. We present an algorithm that may be useful to physicians in determining whether cannabis might be recommended as a treatment in jurisdictions where such use is permitted.
Cannabis is suggested as a treatment option for neuropathic pain, where standard Rx treatments fail, and the risk of substance abuse and mood disorders is judged to be acceptably low. I like that patient preference for oral versus smoked versus vapourised marijuana is part of the treatment too.
Concerns have long been voiced that rapid tolerance to adverse effects might portend tolerance to beneficial effects. Data from studies using oral sprays of cannabinoids or dronabinol in multiple sclerosis report that individuals can reduce the incidence and severity of adverse effects by downward self-titration without loss of analgesia. Other studies in this population note that overall the incidence and severity of adverse effects diminishes over time without evidence of tolerance to analgesic effects. Yet it is rare that clinical trials of cannabinoids extend follow-up beyond 12 weeks, leaving questions on maintenance of gains or need for dose escalation unanswered. One study with 12-month follow-up concluded there may be sustained analgesia for pain associated with multiple sclerosis, where about 30% of cannabinoid-treated participants report continued “improvement” at 12 months compared to about 15% on placebo on doses conservatively limited to a maximum of 25mg THC daily. This suggests that pain relief may be sustained without dose increases. But the study design was not intended to determine the proportion of patients who experienced diminution of effect, or whether dose escalation, even within the set boundary, was needed for maintenance of efficacy.
This is pretty suprising. I don't think one can say the same about the other "habit forming" drugs, esp. opioids and benzodiazepines.
Also:
The classification of marijuana as a Schedule I drug as well as the continuing controversy as to whether or not cannabis is of medical value are obstacles to medical progress in this area. Based on evidence currently available the Schedule I classification is not tenable; it is not accurate that cannabis has no medical value, or that information on safety is lacking. It is true cannabis has some abuse potential, but its profile more closely resembles drugs in Schedule III (where codeine and dronabinol are listed). The continuing conflict between scientific evidence and political ideology will hopefully be reconciled in a judicious manner [...]
Sick burn, guys.

 and the effects are so different! ha
and the effects are so different! ha