-
Psychedelic Drugs Welcome Guest
PD's Best Threads Index Social ThreadSupport Bluelight Psychedelic Beginner's FAQ -
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Miscellaneous The Big & Dandy Psychedelics of the Future Thread
- Thread starter Psychedelics_r_best
- Start date
Have any 7-substituted tryptamines been investigated? these seem promising, as the 7th position corresponds where a MeO group would be in the 2c-x series (the other position being the 4 position which has shown activity with MeO and OH groups)
fastandbulbous
Bluelight Crew
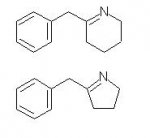
wungchow said:or this guy (mdma substitute)
NO a keto group on a benzene ring is effectively a ring hydroxy and they don't do it because of BBB problems (and possible neurotoxicity). The other two you suggested are imines that easily break down to the ketone and as such do fuck all (except smell funny!)
nuke
Bluelighter
- Joined
- Nov 7, 2004
- Messages
- 4,191
What about 4-propionoxy tryptamines? Even if the geometry was too fudged up to bind correctly and yield activity after getting across the BBB, the propionoxy group would get hydrolysised off yielding the 4-HO-T... If that were to happen, and you made 4-PrO-DMT, you might get something identical to psilocybin.
Last edited:
Helios.
Ex-Bluelighter
to answer the original question,
how about all of your mamas?
how about all of your mamas?
nuke said:n-acetyl and n-propionyl mescaline. Easily and cheaply produced and should be readily hydrolysised in the body to mescaline.
the N acetyl derivative of mescaline is also a metabolite of mescaline. it is however inactive at pretty large doses, I forget the details but it is inactive at doses where mescaline would be very much active.
Prodrug derivatives of phenethylamines are difficult because plain fatty acid amides are simply too stable to hydrolyse in vivo. practical possibilities include hemisuccinylamides or 2-hydroxybenzamides whose rate of hydrolysis is enhanced by intramolecular reactions.
carbamate based prodrugs are also a bit too stable and some trickery has to be employed in order to get a derivative that is stable in the bottle but unstable in the body.
the dihydro n-methyl nicotinamide derivatives are interesting these are able to readily penetrate the blood brain barrier and once inside the brain these are oxidised to the fully aromatic nicotinamide which is readily hydroysed. this is the basis of brain specific prodrugs invented by Bodor.
Peptide derivatives (phenylalanine and lysine being quite readily cleaved)
the real downside is that essentially all these derivatives require the illegal amine to be synthesised as an intermediate (unless the chemist thinks laterally), and if the amine is not illegal then why synthesise the prodrug?
Helios.
Ex-Bluelighter
N-methylmescaline?
(just a guess)
(just a guess)
MDPVagrant
Bluelighter
I don't have much to add to the thread, except to say we could really use some new stimulants (that don't have psychedelia as their main focus). M1 and MDPV are nice, but frankly are getting old...
Helios.
Ex-Bluelighter
bk-TMA
bk-mescaline
bk-mescaline
Helios. said:bk-TMA
bk-mescaline
these molecules are not stable enough to be psychedelic ... Maybe N-Acetyl,b-oxo-phenethylamines ?
Morninggloryseed
Bluelight Crew
^^^^
At the 2006 LSD conference, Shulgin discussed the beta-keto analogues of the 2C family. I did not hear this first hand, so I do not know which ones he tried...I think bk-2C-B was one...but they certainly are active. Just very difficult to make.
At the 2006 LSD conference, Shulgin discussed the beta-keto analogues of the 2C family. I did not hear this first hand, so I do not know which ones he tried...I think bk-2C-B was one...but they certainly are active. Just very difficult to make.
Refluxer
Bluelighter
- Joined
- Feb 15, 2006
- Messages
- 362
MDPVagrant said:I don't have much to add to the thread, except to say we could really use some new stimulants (that don't have psychedelia as their main focus). M1 and MDPV are nice, but frankly are getting old...
I think that is exactly what will happen more than other in 2007. Question is how commercialized they will be. It's good stimulants are my cup of tea.
MDPVagrant said:I don't have much to add to the thread, except to say we could really use some new stimulants (that don't have psychedelia as their main focus). M1 and MDPV are nice, but frankly are getting old...
this is the psychedelic section isn't it?8(
MDPVagrant
Bluelighter
Ya true, I didn't notice (I usually browse by hitting "new posts", not by forum). Anyway I still think we could use more non-psychedelic RC's, no matter what forum it's said in.trip.more said:this is the psychedelic section isn't it?8(
haribo1
Ex-Bluelighter
- Joined
- Nov 29, 2006
- Messages
- 4,826
Dr.Heckyll said:To hell with all these boring phenethylamines, I want to see some Lysergic Acid Amides on the market!
8 ethyl S,S 2,4 dimethyl azetadine amide.
nuke
Bluelighter
- Joined
- Nov 7, 2004
- Messages
- 4,191
Jenkies Batman, what do substituted cyclic propylamines have to do with ergolines???
edit: Rhodium told me all about it,nevermind. That still doesn't help much though, as the everlasting problem is in a synthesis that bypasses lysergic acid/ergotamine somehow.
edit: Rhodium told me all about it,nevermind. That still doesn't help much though, as the everlasting problem is in a synthesis that bypasses lysergic acid/ergotamine somehow.
Last edited: