Welcome to the Big & Dandy AL-LAD Thread - Part 1
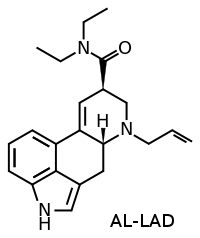
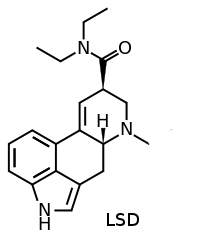
AL-LAD vs. LSD
formula = 6-allyl-6-nor-lysergic acid diethylamide
<< GO TO NEXT BIG & DANDY AL-LAD THREAD (PART 2) >>

Description:
AL-LAD is an alkenated / alkylenated analogue of (6-nor-)LSD, or differently put a 'homologue' of DMT, that has a slightly lower potency in practice and somewhat shorter duration of effects than LSD although it showed higher potency in theory. It gained recognition after Alexander Shulgin described the substance in the book TIHKAL, although David Nichols and Albert Hofmann described AL-LAD 12 years before that in an article. However until recently AL-LAD has not been known to have been available on the market.
Because it is not an alkyl analogue it bypasses UK drug analogue laws. In this early stage of availability on the market AL-LAD is quickly gaining popularity, having a lot of the virtues of LSD and even advantages over it, although opinions and reactions always vary as per YMMV.
Until recently history of use in humans was quite limited, and positive reports do not guarantee physical safety. If it has remotely the therapeutic index LSD has it should be considered physically quite safe, but has not yet been proven, as far as I know.
AL-LAD Blotter Print (depicting structural formula / name):
NSFW:


Ehrlich Reagent Test Color Results:
NSFW:


Wiki & Analysis Data:
NSFW:
Brief overview - What is AL-LAD?
AL-LAD novel hallucinogenic developed originally by David E. Nichols and popularized by Alexander Shulgin. It closely resembles LSD in chemical structure and has very similar dosages and effects.
Chemical and physical properties
AL-LAD is a6-alkylatedanalogue of nor-LSD. (mod edit: the allyl group is an alkylene, not an alkyl because of the double bond)
Effects
Effects can be felt within a quarter hour to an hour or more. Physically the experience can include a warm body high, elevated heart rate and increased energy. Visually the effects are similar to that of LSD (depending on the individual). Users often report euphoria and music enhancement. Some users report a merge between audio and visual aspects of the experience.
Pharmacology, toxicity and general safety
DOSAGE : 80 - 160 micrograms +.
DURATION : 6 - 8 hours.
Very little is known at this stage about the toxicity of AL-LAD and potential long-term effects. As with any RC, caution should be exercised. No deaths or hospitalizations have been reported due to it's usage so far (august 2013) there is no data for lasting negative physical effect.
AL-LAD is every bit as much as potent as it's sister molecule LSD and should be treated with equal respect. This chemical is a powerful psychedelic and the effects are mostly mental/visual. Most online reports have been positive with no complaints of negative physical effect after the experience has ran its course.
Bad trips are always possible with almost any psychedelic and AL-LAD is no exception, with reports mentioning things becoming much too intense and 'cartoon like' at higher dosages. Always make sure you know how much you are ingesting and do so in a safe environment where you feel comfortable. NEVER ingest a psychedelic if you will be working or driving.
There scarce data regarding the combination of AL-LAD and other substances. Combination with MAOIs (monoamine oxidase inhibitors) carries the same risk as with many other psychedelics, however since this combination has not been documented it can NOT be recommended at this point.
Dosages and consumption methods
AL-LAD is easily dissolved onto blotters or can be taken in suspension, held under the tongue or on the gum and cheek. Anecdotal reports mention this compound being active orally, unlike LSD itself. A mild dose can be as low as 80 micrograms, with a intermediate dosages around 150-200 micrograms. Higher dosages have been reported up to 300 and 400 micrograms.
History of usage
There is not a much credible data because of the scarcity of this compound, however Alexander Shulgin has written an extensive report of his own self experimentation in TIHKAL entry #1.
Analysis of AL-LAD
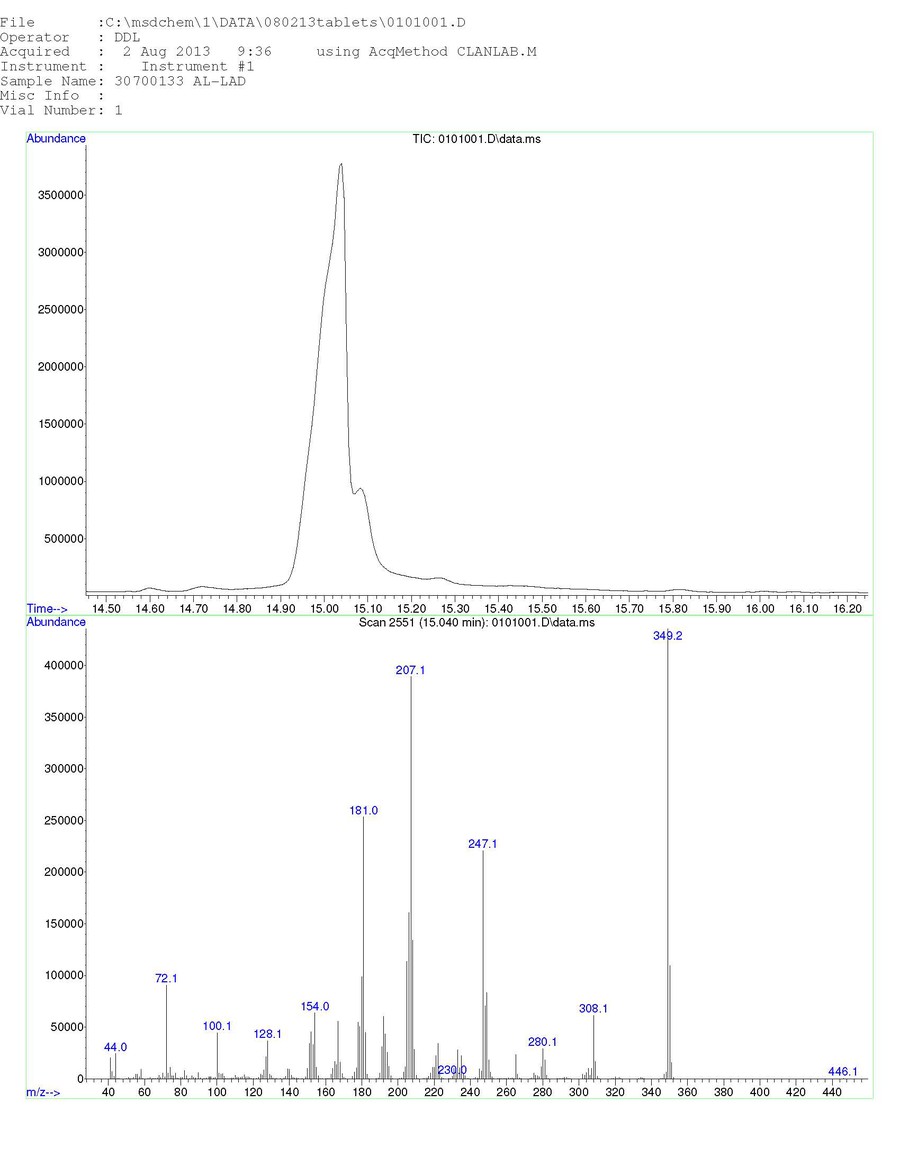

AL-LAD Trip Reports sorted by ascending dosage:
- Bluelight / copy from other forum:
- My first real taste of Al-LAD (unknown dose AL-LAD)
- Lovely on the Sweet Spot (75 ug AL-LAD with GHB and lisdexamphetamine) (erowid link)
- Very Promising (90 ug AL-LAD)
- Untitled report by 'zombywoof' (150ug AL-LAD)
- Lime Green Acid (150 ug AL-LAD)
- Untitled report by 'chinacat' (230 ug AL-LAD)
- Untitled report by 'velmwend' (300 ug AL-LAD)
- Changed my life for the better (300 ug AL-LAD)
- Untitled report #1 - copied from other forum (300 ug AL-LAD with 25B-NBOMe)
- Myself, the Mistral and Kipple (450 ug AL-LAD)
- Where have you been all my life? (450 ug AL-LAD)
- Untitled report #2 - copied from other forum (500 ug AL-LAD)
- Untitled report #3 - copied from other forum (750 ug AL-LAD)
- Answer to all questions (1500 ug AL-LAD)
- Erowid trip report:
- On the Verge (95 ug AL-LAD)
- A Good Intro to an Amazing Compound (150 ug AL-LAD with Cannabis)
- Better than LSD (150 ug AL-LAD with Cannabis)
- Like in a Fairy-Tale (150 ug AL-LAD with LSD)
- The Cutting Edge of Psychedelic Research (187 ug AL-LAD with Cannabis)
- Sedona Sunshine (225 ug AL-LAD)
- The Breaking of Reality (300 ug AL-LAD with Cannabis)
External Links:

Excerpt from TIHKAL Entry:
TIHKAL said:#1. AL-LAD
SYNTHESIS: To a solution of 66 mg nor-LSD (see under ETH-LAD for its preparation) in 2 mL freshly distilled DMF under a nitrogen atmosphere, there was added 48 mg anhydrous K2CO3 and 30 mg allyl bromide. When TLC analysis indicated that the nor-LSD had been consumed (30 min) all volatiles were removed under a hard vacuum. The residue was solubilized in CHCl3 (5x5 mL) and the pooled extracts dried over anhydrous Na2SO4 which was remove by filtration. The filtrate removed under vacuum leving a residual white solid. This was separated into two components by centrifugal chromatography (alumina, CH2Cl2, nitrogen and ammonia atmosphere), the first of which was the major product. After removal of the solvent, this was dissolved in hot benzene, filtered and cooled. The addition of hexane prompted crystallization of AL-LAD (N-allyl-nor-LSD) as a white crystalline product weighing 66 mg, yield 88%. It had a mp of 88-90°C and an [a]D + 41.8 (c 0.44, EtOH).
DOSAGE : 80 - 160 micrograms
DURATION : 6 - 8 h.
QUALITATIVE COMMENTS : (with 50 µg) "I am aware in twenty minutes, and am into a stoned place, not too LSD like, in another hour. I would very much like to push higher, but that is not in the cards today and I must acknowledge recovery by hour eight."
(with 80 µg) "I had a mild effect, although the doors to my repressed feelings somehow really become opened up. There was nothing transcendental here, but there were moments where I felt a conscious separation from the world about me. None of the profound meanings that I had hoped to have explained were explained."
(with 150 µg) "I felt it in less than a quarter hour, and was shooting up past a +++ in another quarter hour. Fast. Just like LSD but without the vaguely sinister push. A little time slowing, randy, no body disturbance. Dropping at six hours and totally tired and going to sleep at twelve hours. I will repeat."
(with 150 µg) "Simply beautiful. Erotic and music absorption after second hour. Clear thinking with superb imagery and good interpretation. Easy, gentle sleeping. Next day -- serene, clear-thinking peacefulness. One of the best materials ever."
(with 160 µg) "I took 160 ug at 11 AM on an empty stomach and lay down to listen to a hypnotic relaxing tape, with eye shades and headphones. The onset was very gradual over two or three hours. There was some visual distortion similar to LSD, but mild. I decided that this was about as intense as it was going to get, so I lay down in the living room with the others. The experience continued to intensify over the next hour in intermittent waves. I had to verify that I was actually in a physical room rather than in the music I was hearing. There was never any fear or panic, but I chose to retreat to a private place for the next couple of hours. Soon I started to feel worse and I tried to gain some insight and relief from my negative attitude. I prayed, and I cried, and I began to feel calmer and had more positive thoughts as to how to deal with the others, but I was still afraid to go out into the group. I was afraid that my hopelessness would bother them but I eventually went back out at about the five hour point and the rest of the day was spent pleasantly and smoothly. I took 2.5 g of L-tryptophan to sleep, and I slept well, waking twice."
(with 160 µg) "I pretreated myself with 40 milligrams of inderal 40 minutes beforehand, took the AL-LAD, and went to bed with eye-shades and ear-phones. There was a very slow onset. The effects were best described as very short bursts of loss of contact with my body, which became increasingly intense and frequent as things progressed. It became really trippy, like acid. There were no visuals with my eyes closed, but when I removed my eye-shades the floors were melting, and the wall patterns and the wood ceiling really flowed. My body felt very blob-like, and I had to get help from my sitter to get up so I could pee. I was very affected by music. There was a very long down-ramp, with physical excitation appearing to linger longer than psychic excitation. Pretty much out of it by 12 hours and I felt well the next day."
(with 200 µg) "This was taken on the tail end (seventh hour) of an MDMA experience. I felt it quickly, but it never got to a super level. Complicated erotic, good talking, looked pretty stoned, and yet I still had cognitive integrity."
EXTENSIONS AND COMMENTARY: This is one of the several very potent compounds in a large series of 5-alkylated analogues of nor-LSD. Most of them proved to be less potent than LSD, and considerably less dramatic. The Inderal mentioned in one of the comments is a trade name for propranolol, an antihypertensive that reduces nervousness.
A comment is appropriate concerning the use of the prefix "nor," as in the name of this material N-allyl-nor-LSD and of its immediate precursor, nor-LSD. Its exact meaning is that there is an alkylated nitrogen atom somewhere that has lost an alkyl group. The original term is from the German phrase "N-ohne-Radical" meaning N (the nitrogen) without the radical (meaning the alkyl group). The removing of the N-methyl group of LSD to form the N-H counterpart is a text book example of this usage. Unfortunately its use has slopped over to embrace the removal of an alkyl group from a heteroatom of any sort. Recently I learned of a metabolite of ibogaine that has lost a methyl group from the indolic oxygen atom (a methoxy has become a hydroxy) and the compound was called noribogaine. The correct term, to retain the use of the parent ibogaine word in its name, would have been desmethyl ibogaine. The removal of something is usually indicated with a "de-" or a "des" prefix ahead of the item that has been lost, as in deoxy (or desoxy) ribonucleic acid, DNA, which is ribonucleic acid (RNA) missing an oxygen atom.
Last edited:




