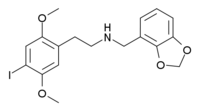
any major dude
Bluelight Crew
I put my solution onto blotter paper then let that sit in my mouth for about 15 minutes. Before this I brushed my teeth thoroughly.
thanks for the info. So that worked pretty well then?
jesus just breathing in the same room as that drug would scare me. :O
yeah, i'm a tad nervous about handling this one as well, but want to try something new, so I may.... still debating it though.
Source is probably from Nichols / Purdue group's work isn't it?
I believe so. Regardless, they were the first to start tacking NBOMe onto various PEA's.