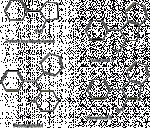Reminisant B
Greenlighter
- Joined
- Nov 3, 2005
- Messages
- 401
Dreaming...
Ok I'm the first to admit I am not the best at this but just thinking aloud I was looking at the structure and comparing it to desoxypipradol...
What do you think of these compounds...
Compound A is similar to 2-aminoindan and the second has an indole. Good possibility / Bad possibility?
I definately like the look of compound A (if its chemically possible)
Ok I'm the first to admit I am not the best at this but just thinking aloud I was looking at the structure and comparing it to desoxypipradol...
What do you think of these compounds...
Compound A is similar to 2-aminoindan and the second has an indole. Good possibility / Bad possibility?
I definately like the look of compound A (if its chemically possible)