AlsoTapered
Bluelighter
- Joined
- Apr 1, 2023
- Messages
- 3,076
Pasteboard - Uploaded Image
Simple and lightning fast image sharing. Upload clipboard images with Copy & Paste and image files with Drag & Drop
pasteboard.co
Library Genesis
The above link confirms that (R)-3,3-dimethylprodine is 7.5-10x morphine in potency.
Library Genesis
The above link confirms that compound 29 has the same potency of pethidine.
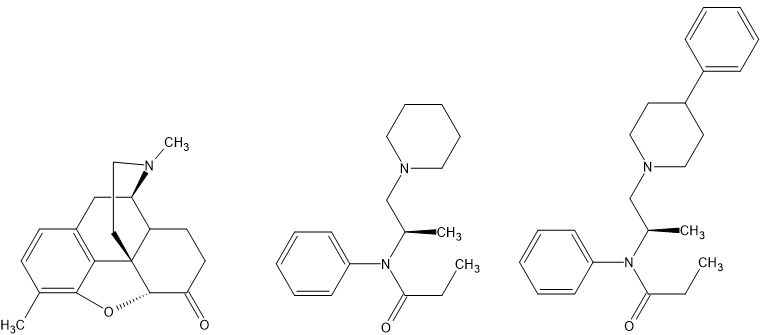
What the above demonstrates is that three compounds that when viewed as CONVENIENT 2D images fails to demonstrate the common core and what constitutes the pharmacophore of mu ligands. These compounds all follow what is commonly known as 'the morphine rune', an early attempt to define structural features that provides affinity:
1-Aromatic System
2-Quaternary Carbon
3-two carbon chain
4-Tertiary amine
While MANY opioids deviate from these rules, it holds true for around 90% of known opioids.
If people wonder why I chose 3,3-dimethyl prodine, it's to demonstrate that a second quaternary carbon increases affinity by the simple expedient of reducing the number of rotatable carbon bonds.
People may well be aware that the potency of both oxymorphone and 3,3-dimethyl prodine may be greatly increased by replacing the N-methyl with an N-(2-phenylethyl) moiety. This is also likely possible for the simplest example by replacing the dimethyl for a 4-phenylpiperidne moiety which overlays the N-pe moiety and indeed closely related compounds are known.
But as an exercise, count how many atoms make up each molecule and thus the MW. You will note that potency is given in mg/kg so the lower the MW of a compound, the more molecules per given mass and so atom for atom the three compounds are closer to being equal in activity.