haribo1
Ex-Bluelighter
- Joined
- Nov 29, 2006
- Messages
- 4,826
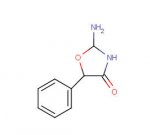
I just wondered if anyone had considered making the ring-substituted permoline series. Benzaldehyde+NaCN->mandonitrile+Guadinine->permoline seems easy enough. The substituted aldehydes can be produced by breaking up the propenyl benzenes so the MD, MM, DMM and so on would be obvious canidates. I don't know where to get 2,5 dimethoxy 4 methyl aldehyde, but I would wager that it would be fair trippy!
Just wondering out loud,
H-)
PS Similarly, the aminorex series could be substituted. In fact, I bet that they would be stronger. After all, aminorex is more active than dexedrine and both isomers are active...
Just wondering out loud,
H-)
PS Similarly, the aminorex series could be substituted. In fact, I bet that they would be stronger. After all, aminorex is more active than dexedrine and both isomers are active...