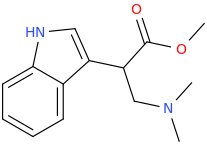
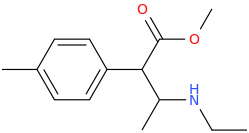
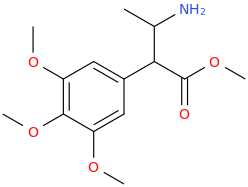
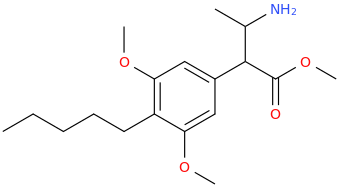
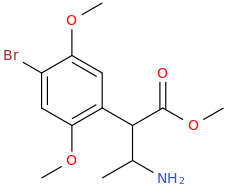
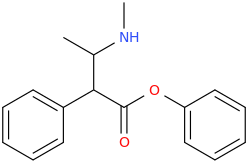
-
Neuroscience & Pharmacology Discussion Welcome Guest
Posting Rules Bluelight Rules Recent Journal Articles Chemistry Mega-Thread FREE Chemistry Databases! Self-Education Guide -
N&PD Moderators: Skorpio | someguyontheinternet
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
🌟🌟 Social 🌟🌟 Rectify's molecular poetry thread
- Thread starter Dresden
- Start date
Rectify
Bluelighter
- Joined
- Oct 20, 2008
- Messages
- 6,936
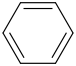
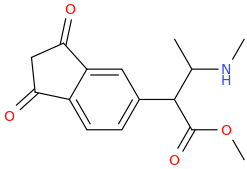
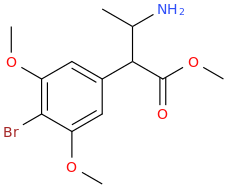
benzene
Cl-(C=O)-CH2-(C=O)-Cl
1,3-dichloroacyl-maleic acid
AlCl3(s)
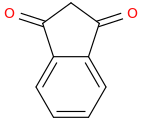
1,3-dioxoindan
Protect oxo's with ethylene glycol, then react with Cl-(C=O)CH2CH3 and AlCl3. Then Br2, then deprotect with H+.
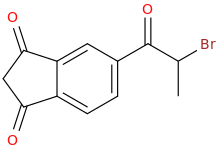
1-(1,3-dioxoindan-5-yl)-1-oxo-2-bromopropane
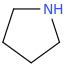
pyrrolidine
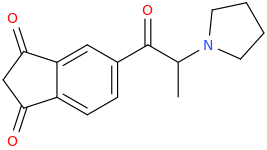
1-(1,3-dioxo-indan-5-yl)-1-oxo-2-(1-pyrrolidinyl)-propane
You might have to fiddle around with the protecting groups, but this is the gist of it.
Last edited:
Rectify
Bluelighter
- Joined
- Oct 20, 2008
- Messages
- 6,936

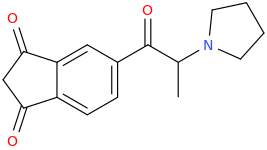
acetylene
Diels Alder
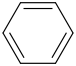
benzene
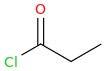
1-chloro-1-oxopropane
AlCl3
Friedel Crafts
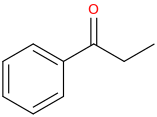
1-phenyl-1-oxopropane
Br2
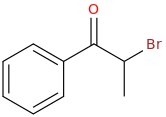
1-phenyl-2-bromo-1-oxopropane
CH3CH2NH2
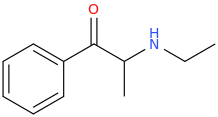
1-phenyl-1-oxo-2-ethylaminopropane
NaBH4
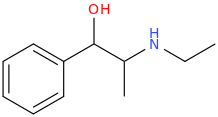
1-phenyl-1-hydroxy-2-ethylaminopropane
Cl-(C=O)-Cl
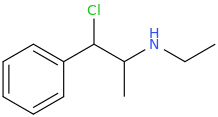
1-phenyl-1-chloro-2-ethylaminopropane
KCN
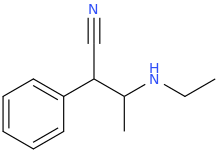
1-phenyl-1-cyano-2-ethylaminopropane
H3O+
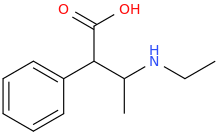
1-phenyl-1-carboxy-2-ethylaminopropane
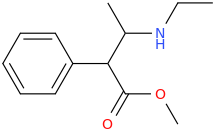
FANCY_FEAST
1-phenyl-1-carbomethoxy-2-ethylaminopropane
Bon apetit!
Last edited:
unodelacosa
Bluelighter
Ah yes, my good ol' buddy of the Mà Huàng persuasion.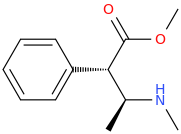
DOUBLE_DEXTER_FINN_AGAIN
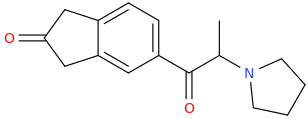
(1S,2S)-1-phenyl-1-carbomethoxy-2-methylaminopropane
from
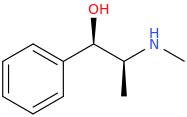
(1R,2S)-1-phenyl-1-hydroxy-2-methylaminopropane
Yes, starting from the (1R,2S)-configuration, the hydroxyl group is converted to a leaving group, such as a mesylate or tosylate. But it's switched back during the second substitution that reacts the resulting compound from the first step with… well I guess something like, α-methoxyacetyl chloride, introducing the carbomethoxy group, no? I'm speculating on the synthetic route a bit here.Actually, I think the hydroxyl group may switch around the stereochemistry of the one spot after it undergoes a substitution reaction.
Pseudoefffffffiiiiii mean pseudomàhuàng, IIRC. Hmm, I'm reminded of PPA and Aminorex synthesis at the moment.Anyway, if that is the case, then this is the correct precursor:
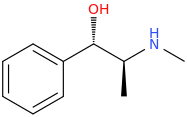
(1S,2S)-1-phenyl-1-hydroxy-2-methylaminopropane
Right? That's how I see it, too. One would start from the (1R,2S) config, and not the pseudo one, (1S,2S).BUT it undergoes 2 substitution reactions, so the former would be correct.
Forgive me for being pedantic and splitting hairs a bit here… The pair of enantiomers with the stereochemistry (1R,2S) and (1S,2R) is traditionally designated "ephedrine", while the pair of enantiomers with the stereochemistry (1R,2R) and (1S,2S) is called "pseudoephedrine". I know you know this already, but I figured it would hurt to post as a reminder to anyone reading this thread… who probs know it too, but … it's in the interests of science and, by extension perhaps if not directly, harm reduction.Errg, Forget Stereochemistry! All I Know Is That Ephedrine Comes In 4 Stereochemicals, And One Of Them Is Correct. Specifically, It Has A (2S).
unodelacosa
Bluelighter
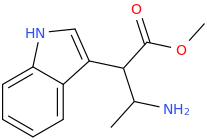
Probably weak as a stimulant, you're saying, right? And yeah I can't imagine that n-substituted ethylhydroxy bond would do much to help w/potency or even perceptible activity without super high/dangerous doses.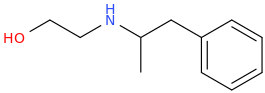
ALCOHOLIC_AMPHETAMINE
N-(2-hydroxyethyl)-1-phenyl-2-aminopropane
Probably weak. I don't know.
Rectify
Bluelighter
- Joined
- Oct 20, 2008
- Messages
- 6,936
I always thought it depended on whether the ephedrine came from ma huang or which brand of cold pills that were used.
I know there are 2^n where n = 2 chiral centers means that there are 4 possible stereoisomers of ephedrine.
Interestingly, I have found that methamphetamine made from ma huang extract is smoother and weaker than that made from cold pills. I don't know why.
But believe it or not, I am not a meth chef.
I know there are 2^n where n = 2 chiral centers means that there are 4 possible stereoisomers of ephedrine.
Interestingly, I have found that methamphetamine made from ma huang extract is smoother and weaker than that made from cold pills. I don't know why.
But believe it or not, I am not a meth chef.
Rectify
Bluelighter
- Joined
- Oct 20, 2008
- Messages
- 6,936
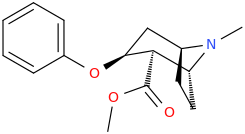
JASON_CHRISTOPHER
methyl (1R,2R,3S,5S)-3-(phenoxy)-8-methyl-8-azabicyclo[3.2.1]octane-2-carboxylate
If I Knew How To Make It, I Would Tell You So.
HO-CH2CH2-O-CH2CH2-OH
JAMES
Stronger Than It Looks
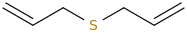
HUFFABLE_ALLICIN_WUNDERLANDE.
diallylsulfur
Last edited: