Mr. Krinkle
Bluelighter
- Joined
- Apr 2, 2005
- Messages
- 20,883
The antidepressant properties of hallucinogenic drugs may stem from their ability to bind to intracellular serotonin receptors, a study suggests.
Besides inducing “trips,” psychedelics such as LSD—administered in parallel to psychological support—have been shown to have beneficial effects in cases of depression, anxiety, and addiction. Although these clinical observations tend to be weakened by the absence of adequate placebo-controlled conditions, several research groups are joining efforts to understand how these drugs can sometimes surpass common antidepressants in efficacy and speed.
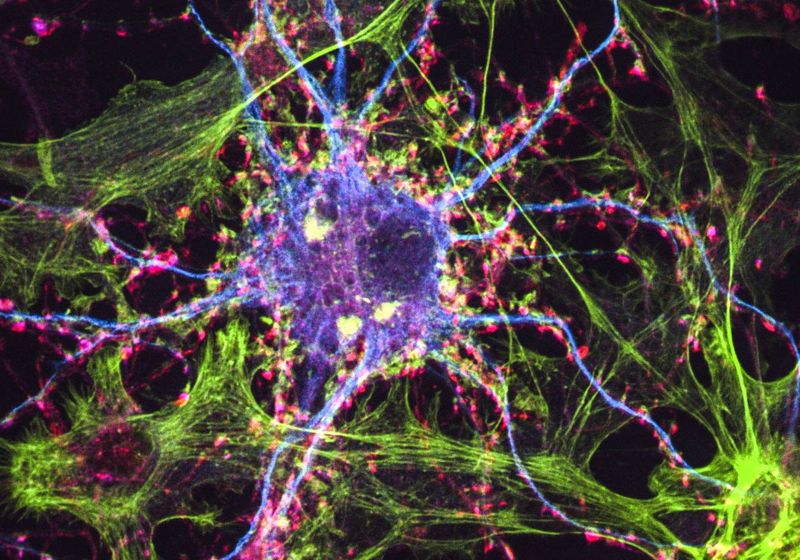
Psychedelics Slip Past Cell Membranes When Treating Depression
The antidepressant properties of hallucinogenic drugs may stem from their ability to bind to intracellular serotonin receptors, a study suggests.