paracelsius
Bluelighter
- Joined
- Mar 11, 2020
- Messages
- 197
I have been looking at human 5HT2A crystal structures complex with psychedelics, from that Chinese x-ray Science paper of 2A with hallucinogenic (psilocin, LSD) and non-hallucinogenic (lisuride) tryptamines.
Well that study (I mean the X-ray structure) doesnt really tell you how a given agonist binding may lead to (selective) activation of one or the other pathways(or both) and lead to hallucinogenic effects (rather HTR) or not. I dont buy into the rational they proposed tho, I mean the structural basis for functional selectivity leading to hallucinogenic effect (or not) of a 5HT2A agonist.
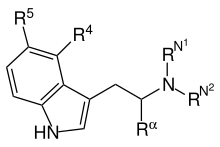
Here is psilocin bound to human 5HT2A receptor (same ref; pdb code 7wc5 ). Processed to zoom on the active site details. 5-MeO-DMT is superimposed to psilocin (not optimized!) for comparison. Also substituted tryptamines structure (with atom numbering) from wikipage. well not quite fully numbered but notice the 4,5,6 and 7 position on the benzene ring.
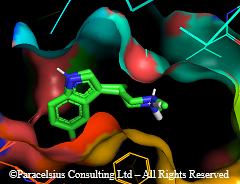
If you actually look at the structure, it is easy to see rational for the SAR of psychedelics, at least tryptamine-types. A variety of substituents on the amine N can be tolerated. There is lots of empty space on that pocket of the receptor binding site (bottom-right corner in pic). Mostly large hydrophobic. You can fit variety of N-substitutents, even large ones like a benzyl to optimize fitting/binding: No wonder Nbome, N-benzyltryptamine, even N-Bromobenzyl are super-potent (fit better). As well as a variety of N,N-dialkyltryptamines made by Shulgin (DET, MET, DPT, MiPT, DiPT....etc).
View from indole phenyl side:
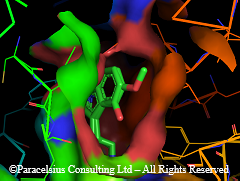
R5 Substituents at the 5-position (like Methoxy in 5-MeO-DMT or 5MeO-DiPT aka foxy...etc) fit nicely which explain why 5-sub tryptamines are more potent in general. So is the 4-sub and 7-sub positions but not the 6-position, there is just no space to accommodate any thing larger than a H at 6-position without paying energy penalty, maybe a Fluoro. That explains why 6-MeO-DMT is like 800x less potent than 5-MeO-DMT and is non-hallucinogenic.
Now that doesnt really tell you much about what may happen next, once the drug binds the receptor, ie which downstream signalling is selectively activated -> hallucinogenic or not, even type of “hallucinations”(visual, auditory, DMT entities, DPT religious theme..etc etc. Only that the designed drug may bind and activate the 2A receptor, predicting what happen next is the million$ question.
The interesting thing tho, the psychedelic effects can be dramatically different depending on the substituent. Like take Dipropyltryptamine DPT (one I always wanted to try), well that compound is purely auditory while some other tryptamines are pretty visual. I mean it is pretty hard to predict that from the drug chemical structure, kind like draw a Structure-Hallucinations-Relationship (SHR) of psychedelics! would be nice to see that tho: Structure-Hallucinations-Relationships of Psychechedelic Tryptamines!..anyway
If I was to design new psychedelics, I would focus on the N-subs and/or 7-substitued tryptamines. Shulgin made a bunch of N-sub-Tryptamines, but mostly N,N-disubstituted. Not so much mono-subs and 7-subs tryptamines (iirc only N-ethyltryptamine NET was mentioned in TIHKAL). But I believe there is lots to explore especially 7-subs tryptamines. Lots of tryptamines with interesting profile are yet be discovered. They certainly patentable too....hope you enjoy reading Good'day All BLighters.. You'all Stay Safe Out There.
EDIT: please note the imgs are © DM me if you intent to use, thx.
Structure-based discovery of nonhallucinogenic psychedelic analogs
Cao et al... Science 2022, 375(), 403-411. DOI: 10.1126/science.abl861
Abstract
Drugs that target the human serotonin 2A receptor (5-HT2AR) are used to treat neuropsychiatric diseases; however, many have hallucinogenic effects, hampering their use. Here, we present structures of 5-HT2AR complexed with the psychedelic drugs psilocin (the active metabolite of psilocybin) and d-lysergic acid diethylamide (LSD), as well as the endogenous neurotransmitter serotonin and the nonhallucinogenic psychedelic analog lisuride. Serotonin and psilocin display a second binding mode in addition to the canonical mode, which enabled the design of the psychedelic IHCH-7113 (a substructure of antipsychotic lumateperone) and several 5-HT2AR β-arrestin–biased agonists that displayed antidepressant-like activity in mice but without hallucinogenic effects. The 5-HT2AR complex structures presented herein and the resulting insights provide a solid foundation for the structure-based design of safe and effective nonhallucinogenic psychedelic analogs with therapeutic effects.
Well that study (I mean the X-ray structure) doesnt really tell you how a given agonist binding may lead to (selective) activation of one or the other pathways(or both) and lead to hallucinogenic effects (rather HTR) or not. I dont buy into the rational they proposed tho, I mean the structural basis for functional selectivity leading to hallucinogenic effect (or not) of a 5HT2A agonist.
Here is psilocin bound to human 5HT2A receptor (same ref; pdb code 7wc5 ). Processed to zoom on the active site details. 5-MeO-DMT is superimposed to psilocin (not optimized!) for comparison. Also substituted tryptamines structure (with atom numbering) from wikipage. well not quite fully numbered but notice the 4,5,6 and 7 position on the benzene ring.


If you actually look at the structure, it is easy to see rational for the SAR of psychedelics, at least tryptamine-types. A variety of substituents on the amine N can be tolerated. There is lots of empty space on that pocket of the receptor binding site (bottom-right corner in pic). Mostly large hydrophobic. You can fit variety of N-substitutents, even large ones like a benzyl to optimize fitting/binding: No wonder Nbome, N-benzyltryptamine, even N-Bromobenzyl are super-potent (fit better). As well as a variety of N,N-dialkyltryptamines made by Shulgin (DET, MET, DPT, MiPT, DiPT....etc).
View from indole phenyl side:

R5 Substituents at the 5-position (like Methoxy in 5-MeO-DMT or 5MeO-DiPT aka foxy...etc) fit nicely which explain why 5-sub tryptamines are more potent in general. So is the 4-sub and 7-sub positions but not the 6-position, there is just no space to accommodate any thing larger than a H at 6-position without paying energy penalty, maybe a Fluoro. That explains why 6-MeO-DMT is like 800x less potent than 5-MeO-DMT and is non-hallucinogenic.
Now that doesnt really tell you much about what may happen next, once the drug binds the receptor, ie which downstream signalling is selectively activated -> hallucinogenic or not, even type of “hallucinations”(visual, auditory, DMT entities, DPT religious theme..etc etc. Only that the designed drug may bind and activate the 2A receptor, predicting what happen next is the million$ question.
The interesting thing tho, the psychedelic effects can be dramatically different depending on the substituent. Like take Dipropyltryptamine DPT (one I always wanted to try), well that compound is purely auditory while some other tryptamines are pretty visual. I mean it is pretty hard to predict that from the drug chemical structure, kind like draw a Structure-Hallucinations-Relationship (SHR) of psychedelics! would be nice to see that tho: Structure-Hallucinations-Relationships of Psychechedelic Tryptamines!..anyway
If I was to design new psychedelics, I would focus on the N-subs and/or 7-substitued tryptamines. Shulgin made a bunch of N-sub-Tryptamines, but mostly N,N-disubstituted. Not so much mono-subs and 7-subs tryptamines (iirc only N-ethyltryptamine NET was mentioned in TIHKAL). But I believe there is lots to explore especially 7-subs tryptamines. Lots of tryptamines with interesting profile are yet be discovered. They certainly patentable too....hope you enjoy reading Good'day All BLighters.. You'all Stay Safe Out There.
EDIT: please note the imgs are © DM me if you intent to use, thx.



