Billowtasters
Greenlighter
- Joined
- Jan 4, 2023
- Messages
- 18
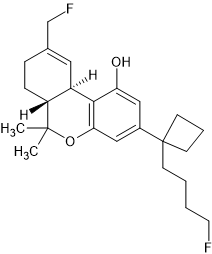
This substance is very nice. Get a hot warm fuzzy feeling that lasts about 2 hours before it slowly fades off over the next 2-3 hours.
Confidence is boosted, mood is elevated. Pain is taken away.
I first attempted with a nasal spray however being extra cautious I made the spray a bit too weak and required many a squirts to build up to the opiate warmth.
I then decided the next day to simply snort some with an eye balled amount as the scales would not pick it up. I do not revoke d this but I had my batch tested and it came up as about 40% pure. This would be low for most drugs but for this drug I am grateful for it.
It is very fine yellow crystals.
The one thing that I liked most was my ability to urinate with ease 4 hours after dosing. One of the main reasons for me disliking other opiates.
Is it addictive? Of course. Please do not try this drug unless you are very experienced.
Confidence is boosted, mood is elevated. Pain is taken away.
I first attempted with a nasal spray however being extra cautious I made the spray a bit too weak and required many a squirts to build up to the opiate warmth.
I then decided the next day to simply snort some with an eye balled amount as the scales would not pick it up. I do not revoke d this but I had my batch tested and it came up as about 40% pure. This would be low for most drugs but for this drug I am grateful for it.
It is very fine yellow crystals.
The one thing that I liked most was my ability to urinate with ease 4 hours after dosing. One of the main reasons for me disliking other opiates.
Is it addictive? Of course. Please do not try this drug unless you are very experienced.