-
Neuroscience & Pharmacology Discussion Welcome Guest
Posting Rules Bluelight Rules Recent Journal Articles Chemistry Mega-Thread FREE Chemistry Databases! Self-Education Guide -
N&PD Moderators: Skorpio | someguyontheinternet
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
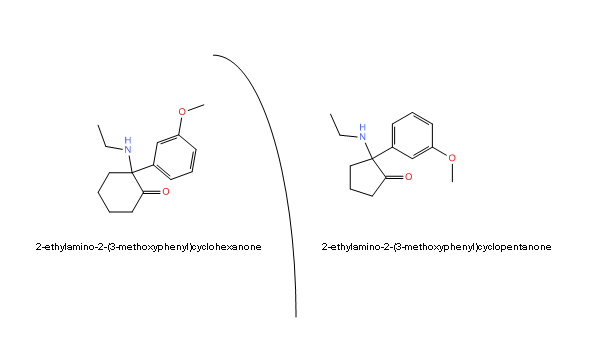
Possible CNS activity of a major methoxetamine synthesis impurity?
- Thread starter klfiend
- Start date
A significant amount of a by-product (impurity) was
produced during the synthesis of methoxetamine.
The article never states that they actually found this particular impurity in MXE in the field. It just says that during the DEA's synthesis, they accidentally made this byproduct. We don't know if the "UK" suppliers or the Chinese manufacturers use the DEA's synthesis protocol or totally different ones. Until someone finds this impurity in commercial MXE, all we can do is speculate.
Last edited:
Hammilton
Bluelighter
- Joined
- Sep 2, 2008
- Messages
- 3,435
Can't find anything in the literature having that cyclopentane ring, interesting.
Perhaps not a close ketamine analogue, but if I'm not mistaken, the PCP analogue with a cyclopentane in place of cyclohexane is actually a scheduled drug in the US.
atrollappears
Bluelighter
Which one is soluble in acetone, MXE or the impurity?
both as the hcl salt are likely to have the same solubility in any given solvent, 'washing' unidentified white powders in acetone that hasn't been dried over silica or MgSO4 etc isn't really helpful for anything, like I said it would be much more informative to TLC samples from several differing batches of 'mxe' together on a single plate.
edit> then if there was distinct seperation of more than one spot with any particular sample, the same material could then be seperated on a silica column, recrystallised and sent for NMR / MS to identify for sure what it was, that is if anyone cared enough to go to the effort...
edit> then if there was distinct seperation of more than one spot with any particular sample, the same material could then be seperated on a silica column, recrystallised and sent for NMR / MS to identify for sure what it was, that is if anyone cared enough to go to the effort...
Last edited:
atrollappears
Bluelighter
^Well as someone quoted earlier in the thread, the DEA study found that the two can be separated with an acetone wash. And I wasn't asking so I could measure the purity, I was asking so I could purify a sample.
Small (milligram) amounts could be seperated with inexpensive TLC plates (cutting out seperated spots), larger amounts with preparative TLC plates, even larger amounts with straightforward flash chromatography using a silica column.
With any unidentified white powder, you can't really make presumptions about what it might contain, e.g wether it contains methoxetamine hcl and the impurity mentioned, multiple organic reaction impurities, diluents, adulterants and so on, without doing some sort of rudimentary analysis first.
It's difficult to generalise about any particular sample one might come across, that particular impurity could be solely confined to one particular batch produced by any number of labs, and once the sample in question gets into ones hands it could have been through any number of intermediaries that could have added adulterants or diluents or stored the material in a way that caused decomposition products to appear and so on...
With any unidentified white powder, you can't really make presumptions about what it might contain, e.g wether it contains methoxetamine hcl and the impurity mentioned, multiple organic reaction impurities, diluents, adulterants and so on, without doing some sort of rudimentary analysis first.
It's difficult to generalise about any particular sample one might come across, that particular impurity could be solely confined to one particular batch produced by any number of labs, and once the sample in question gets into ones hands it could have been through any number of intermediaries that could have added adulterants or diluents or stored the material in a way that caused decomposition products to appear and so on...
Last edited:
nuke
Bluelighter
- Joined
- Nov 7, 2004
- Messages
- 4,191
As everyone said, someone with a bunch of methoxetamine should just run some TLCs. TLC plates aren't controlled and can be ordered from a ton of places. I'd guess you can run it in 50:50:1 xylene : acetone : glacial acetic acid with good resolution on a full sized plate so long as you spot the free bases.
edit: Well, I guess the obvious problem is that even if you spot the free base it'll be quickly protonated by the GAA, which you may have to skip on.
edit: Well, I guess the obvious problem is that even if you spot the free base it'll be quickly protonated by the GAA, which you may have to skip on.
Last edited:
I seem to remember doing a test seperation on a 10x5cm UV254nm plate using either an MeOH/IPA/Acetone mixture or one of those on their own, with a couple of drops of household ammonia added, spotting the methoxetamine hcl sample onto the plate after having dissolved it in methanol. I'm pretty sure it either migrated too far up the plate or not far enough to be called an ideal solvent system though, I didn't follow it up and don't have the notes anymore unfortunately.
edit> I think the mix was too polar and I was gonna rerun it with some toluene added.
edit> I think the mix was too polar and I was gonna rerun it with some toluene added.
Last edited:
This paper describes a closely related compound to the one the OP mentions:
Tice, Colin M.; Hormann, Robert E.; Thompson, Christine S.; Friz, Jennifer L.; Cavanaugh, Caitlin K.; Michelotti, Enrique L.; Garcia, Javier; Nicolas, Ernesto; Albericio, Fernando
Bioorganic & Medicinal Chemistry Letters, 2003 , vol. 13, # 3 p. 475 - 478
Synthesis and SAR of α-Acylaminoketone Ligands for Control of Gene Expression
A lead discovery library and a follow-up focused library of α-acylaminoketones were designed based on known dibenzoylhydrazine ecdysone agonists, including GSTM-E. The compounds were assayed in mammalian cells expressing the ecdysone receptor from Bombyx mori for their ability to cause expression of a reporter gene downstream of an ecdysone response element. The most potent α-acylaminoketones were comparable to GSTM-E in this assay.
Tice, Colin M.; Hormann, Robert E.; Thompson, Christine S.; Friz, Jennifer L.; Cavanaugh, Caitlin K.; Michelotti, Enrique L.; Garcia, Javier; Nicolas, Ernesto; Albericio, Fernando
Bioorganic & Medicinal Chemistry Letters, 2003 , vol. 13, # 3 p. 475 - 478
Synthesis and SAR of α-Acylaminoketone Ligands for Control of Gene Expression
A lead discovery library and a follow-up focused library of α-acylaminoketones were designed based on known dibenzoylhydrazine ecdysone agonists, including GSTM-E. The compounds were assayed in mammalian cells expressing the ecdysone receptor from Bombyx mori for their ability to cause expression of a reporter gene downstream of an ecdysone response element. The most potent α-acylaminoketones were comparable to GSTM-E in this assay.
Can anybody gess from the spectra how big the percentage of this is in their methoxetamine?
I'm wondering if this might be responsible for the mania some users report with mxe.
mania is reported with almost every arylocyclohexamines, some more, some less and methoxetamine has a pretty wide spectrum of effects from person to person and seems overall pretty unpredictable in that matter. id rather blame the mania part on the strange nature of this compound than on impurities. i experienced problems with building up mania and i think that means that mxe just isnt the right choice for everyones brain chemisty.
It's impossible to say what might be in your methoxetamine, after reading that DEA paper it doesn't imply that this impurity is going to present in every synth. Off-white colour could just mean the reaction hasn't been worked up properly and purified completely on a column. Guessing just isn't viable.
planckunit
Bluelighter
- Joined
- Aug 8, 2007
- Messages
- 105
Do you think there is also a possibility that the product gets formed at an earlier stage, when the bromide is converted to the N-methylimine. (so alkylation at this step instead)
sn23
Bluelighter
- Joined
- Dec 31, 2010
- Messages
- 239
Do you think there is also a possibility that the product gets formed at an earlier stage, when the bromide is converted to the N-methylimine. (so alkylation at this step instead)
Yep, that's a side reaction I'd expect to occur during imine formation. I guess chromatography is needed to seperate that from methoxetamine (or from the N-ethylimine-cyclopentanol, if they clean up before the heating step) and I'm not sure all manufacturers would go through that hassle.
I don't really see why substantial amounts of this compound should be formed during neutralization of methoxetamine base, as the amine is way more basic than carbonyl-oxygen.
Here's the mass spectrum of a very common impurity in MXE (second graphic), is it 1-(ethylamino)cyclopentyl](3-methoxyphenyl)methanone?
http://www.sendspace.com/file/bd762b
http://www.sendspace.com/file/bd762b
pr0d1gy
Bluelighter
Here's the mass spectrum of a very common impurity in MXE (second graphic), is it 1-(ethylamino)cyclopentyl](3-methoxyphenyl)methanone?
http://www.sendspace.com/file/bd762b
No, that doesn't appear to be 1-(ethylamino)cyclopentyl](3-methoxyphenyl)methanone. That is somewhat interesting though, but with only the GC/MS it is impossible to tell what we are looking at. I'm curious how this impurity was isolated from methoxetamine and if you have any other spectra available.