Terminal Run: Part One
And if you think the Benzyl is potent then you haven't felt what the
para-Fluoro substitution on that Benzyl does for both overall potency and CB1 activity.
That single
para-Fluoro substitution on the Benzyl turns
MDMB-CHMINACA into the stronger
MDMB-FUBINACA which is already showing a superior kill-record:
MDMB-FUBINACA / MDMB(N)-Bz-F / FUB-MDMB
Methyl 2-({1-[(4-fluorobenzyl)methyl]-1H-indazol-3-yl}formamido)-3,3-dimethylbutanoate
As I wrote in the
Rational Cannabinoid Design: From Intoxicant to Chemical Weapon thread: Electronegative substitutions (ie. Fluorination) at the terminus of either the attached pentyl chain or the corresponding cyclic function such as Cyclohexyl or Benzyl enormously increase both overall potency and CB1-selectivity.
MDMB-FUBINACA is not the end of this road: it is only one-third of the way at best to our Final Destination.
The end of this road is maximum electronegativity at the
para/terminus. Terminal electronegativity.
Fluorine is already the most electronegative element in this Universe so the only way to increase the terminal [
no pun intended] electronegativity of a single Fluoro-substituted Cannabinoid is to add more Fluoro-substitutions but
the limit to these substitutions is how many can be accomplished at the terminal end.
Non-terminal Fluoro-substitutions simply draw electrons back the opposite way - working against the goal of drawing as much electron pressure as possible to the terminal end of the Pentyl/cyclic function. At some point the further addition of Trifluoromethyl groups at the
para position will result in a net loss of CB-receptor activation potency due to steric hindrance.
That is achieved for the following cases - please ignore any mistakes on the correspondence between chemical names and today's bizarre synthetic Cannabinoid naming unconventions - the goal is elucidation of structure-function activity relationships:
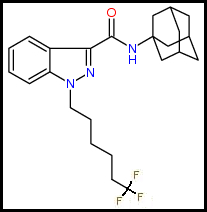
1. Pentyl Chain: Terminal para-Trifluorination: As an example, below is 5F-AKB48 and its para-Trifluoro analog whose potency far exceeds its parent with bonus higher CB1 activity. I apologize for the structural rape of this old and popular Cannabinoid but it has to be done for the sake of a future:
Parent Compound: 5F-AKB48: 1-(5-Fluoropentyl)-N-(tricyclo[3.3.1.1~3,7~]dec-1-yl)-1H-indazole-3-carboxamide
Trifluoro Analog: 5TFM-AKB48: 1-(5-Trifluoropentyl)-N-(tricyclo[3.3.1.1~3,7~]dec-1-yl)-1H-indazole-3-carboxamide:
Parent Compound: FUB-AKB48 (FUB-APINACA): N-(adamantan-1-yl)-1-[(4-fluorophenyl)methyl]-1H-indazole-3-carboxamide
Trifluoro Analog: TFMUB-AKB48 (TFMUB-APINACA): N-(adamantan-1-yl)-1-[(4-trifluoromethylphenyl)methyl]-1H-indazole-3-carboxamide:
2. Cyclohexyl Function: para-Difluorination: Cyclohexyls are saturated having two Hydrogens on each Carbon so there is room for one more para-Fluoro substitution.
Parent Compound: 4F-MDMB-CHMINACA (MDMB N-BZ-F)
Difluoro Analog: 4DF-MDMB-CHMINACA (4DF-MDMB N-BZ-F):
3. Cyclohexyl Function: Additive para-Trifluoromethylation: To maximize terminal electronegativity requires the substitution of Trifluoromethyls for both para-Hydrogens. It should be noted that a double Trifluoromethyl substitution in addition to maximizing terminal electronegativity also introduces significant bulk which could cause steric hindrance at that location. In-vivo testing will be required to determine whether this added bulk causes a net potency losee or gain despite the increased electronegativity.
Parent Compound: 4F-MDMB-CHMINACA (MDMB N-BZ-F)
Trifluoro Analog: 4TFM-MDMB-CHMINACA (4TFM-MDMB N-BZ-F):
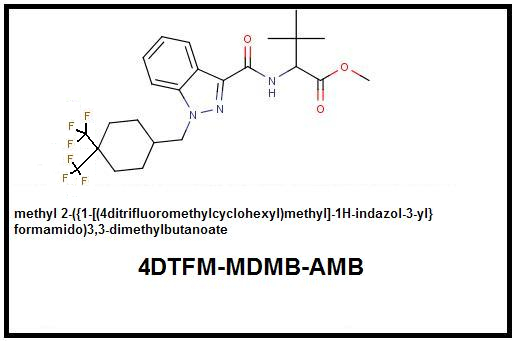
4. Cyclohexyl Function: Additive Double para-Trifluoromethylation: To maximize terminal electronegativity requires the substitution of Trifluoromethyls for both para-Hydrogens. It should be noted that a double Trifluoromethyl substitution in addition to maximizing terminal electronegativity also introduces significant bulk which could cause steric hindrance at that location. In-vivo testing will be required to determine whether this added bulk causes a net potency loss or gain despite the increased electronegativity.
Parent Compound: 4F-MDMB-CHMINACA (MDMB N-BZ-F)
Ditrifluoromethyl Analog: 4DTFM-MDMB-CHMINACA (4DTFM-MDMB N-BZ-F):
5. Benzyl Function: Additive para-Trifluoromethylation: The Benzyl function is limited to a single para-Hydrogen Fluoro-substitution thus it has half the number of higher-electronegativity Fluoro-analogues as corresponding Cyclohexyls do.
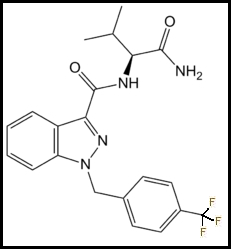
Parent Compound: AB-FUBINACA: N-[(1S)-1-(Aminocarbonyl)-2-methylpropyl]-1-[(4-fluorophenyl)methyl]-1H-indazole-3-carboxamide
Trifluoromethyl Analog: AB-TFMUBINACA: N-[(1S)-1-(Aminocarbonyl)-2-methylpropyl]-1-[(4-trifluoromethylphenyl)methyl]-1H-indazole-3-carboxamide:
A predictable potency-independent and rather ugly result of these further electronegative Fluoro-substitutions is the increase of Fluorotoxicity in users who heat/thermal-vaporize/burn the molecules. At maximum Fluorination the Fluorotoxicity is doubled, tripled or hexed in the case of
additive double para-Trifluoromethylation which introduces two Trifluoromethyl groups providing six Fluorine atoms per molecule.