MDMA neurotoxicity/brain damage
Is MDMA neurotoxic?
by Aimee Sarmiento, PharmD 2021 and Benjamin Malcolm, PharmD, MPH, BCPP | 10 June 2020
Neurotoxicity resulting in low mood, anxiety, insomnia and problems with cognition has been reported and well documented in literature relating to use of ‘Ecstasy’, but what about pure MDMA?
MDMA-induced neurotoxicity use has been the subject of intense controversy and even scandal within the medical community over decades. As MDMA assisted psychotherapy progresses toward approval as a legal therapeutic entity, the questions regarding neurotoxicity of MDMA will be clinically relevant as patients and providers consider risks and benefits of use. Moreover, clinical trials are shedding some light on thresholds for neurotoxic effects and providing high quality data to help guide safe use.
In this post we’ll give some background information on what neurotoxicity is, how MDMA may act as a neurotoxin, summarize research findings on MDMA-induced neurotoxicity, and explain use parameters that are likely to prevent development of significant neurotoxic effects.
“Recommendations” for harm reduction in persons that choose to use MDMA or ‘Ecstasy’ are not intended to condone the use of illicit substances or recommending their use. This article is for information, education, and harm-reduction purposes. The authors recommend you do not break the law.
First of all, what IS neurotoxicity?
Neurotoxicity involves damage to neurons of the central or peripheral nervous system. It can be caused by several things, including drugs. One common misconception is that neurotoxicity exists as an all or nothing event - that is your neurons are either healthy or destroyed, although it is best understood as a symptom spectrum that can be reversible in some cases and irreversible in others. It can begin with small functional deficits, which gradually progress to a larger-scale functional decline or symptoms.
In the case of neurotoxicity as it relates to MDMA, this progression may present itself in an insidious manner, whether it be gradual loss of memory, behavioral and mood issues, or decreased cognitive function over repeated exposures. Of course, large overdoses can produce severe neurotoxicity rapidly. Drugs can produce neurotoxicity through many different types of mechanisms, too many to recount here, although it can be noted that a drug may have a dosing window in which no neurotoxicity is observed due to the dose being modest enough to not push a biological system to such extreme points that toxic responses occur. Even substances we consider completely benign and healthful, such as water, can become neurotoxic under particular circumstances (e.g. drinking 2 gallons in a single hour).
Therefore, our question of MDMA-induced neurotoxicity needs more nuance than ‘is it neurotoxic or not?’ The answer is “yes it is”, but it’s misleading. This is because it inevitably depends on the circumstances of administration. Instead, the better question to ask is ‘under what conditions does MDMA cause neurotoxicity?’
How does MDMA lead to neurotoxicity?
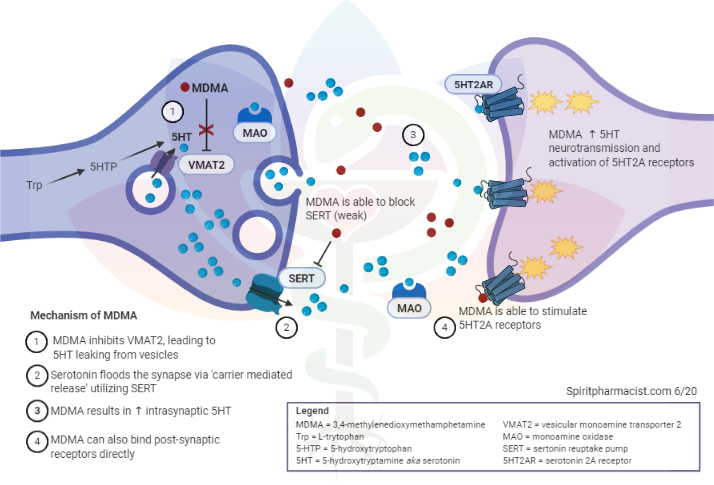
Before discussing how MDMA leads to neurotoxicity, it may be helpful to review how MDMA works. MDMA acts on several neurotransmitter systems, although primarily releases serotonin from presynaptic nerve terminals, resulting in acute depletion of serotonin stores and synaptic flooding of serotonin. This has led researchers to believe neurotoxicity is a consequence of damage to serotonergic neurocircuitry. Subsequently, evidence of neurotoxicity for MDMA is mainly quantified through 5-HT (serotonin) concentrations, activity or presence of enzymes involved with serotonin synthesis or transport (TH and SERT), and visualization of axons immunoreactive for 5-HT or SERT through imaging.
Mechanisms of MDMA-induced neurotoxicity
Sometimes it may be as simple as the dose determining if poisonous or not. With higher doses there is a greater chance of profound serotonin depletion. Frequency of use is also probably consequential as dosing on a weekly basis (as some may be if frequenting raves often) is not enough time to allow for recovery from prior use, even in some taking doses in clinical environments. This is rather simple in that your serotonin neuron simply has a capacity for how high the concentrations can be without damage and how fast it can return to balance after use. The two may go hand in hand as frequent use can produce tolerance and result in increased doses when used.
Other explanations are more complicated. For example, MDMA is metabolized to reactive metabolites such as HHMA or HHA, which can cause cellular damage. The serotonin excess may also lead to lasting reduction in gene expression resulting in lowered expressed of 5HT or SERT. The latter hypothesis has challenges the ‘neurodegenerative’ theory of MDMA induce neurotoxicity which argues for direct damage to serotonin neurocircuits and argues instead for a genetic reason for changes in serotonin function.
It is crucial to note that MDMA’s neurotoxic potential is increased by other substances such as alcohol or amphetamine, which are commonly taken in conjunction with MDMA at nightlife events. Concurrent substances can be ingested either advertently or inadvertently as many Ecstasy tablets contain adulterants or misrepresented substances. For example, one analysis found almost half of ecstasy tablets continued < 67% MDMA and that caffeine and amphetamine were common adulterants. Other common adulterants include novel psychoactive substances such as other phenethylamine (amphetamine) based drugs. Many events sell alcohol or may limit water availability. In combination, both alcohol and MDMA exert neurotoxic effects by impairing the survival of neuronal precursors in the hippocampal dentate gyrus, an area of the brain important for neuronal generation, learning, and memory. Beyond alcohol or amphetamine(s), rave scenes can expose users to myriad other substances, such as GHB (gamma-hydroxybutyrate) or ketamine, which may also increase risk. Recently and tragically, other adulterants from diverse drug classes have began to be found on ecstasy tablets such as fentanyl. Ecstasy is recommended to be tested for presence of desired agent (MDMA) and absence of deadly adulterants (fentanyl) by harm reduction organizations. Further information and testing kits for purchase can be found at dancesafe.org
Other aspects of MDMA use that may contribute to neurotoxicity is the recreational environment and timing of administration. Users may stay up all night on MDMA and related stimulants, which could trigger a sleep deprived state. Sleep deprivation is well known to impair cognition and lower mood, thus plausibly leads to some of the observed week after effects. This issue may be minimized by timing MDMA administration such that the user can fall asleep at a regular time or with minimal disruption to habitual circadian patterns. Another factor that may play a role in toxicity is the bioenergetic stress (namely thermal stress) secondary to MDMA being used in hot environments, raising core body temperatures, and being associated with high output physical activity.
Though the jury is still out on exactly how MDMA exerts its neurotoxic effects, the bottom line is that MDMA has several underlying explanations of why it could be neurotoxic as well as evidence from recreational use environments that it can be neurotoxic under certain circumstances. There circumstances tend to work in conjunction within subcultures that use Ecstasy heavily.
At what point does MDMA become neurotoxic?
The potential for MDMA to act as a neurotoxin is determined by the dose, frequency of exposure, timeline of exposure, concurrent substance use, and overall setting or context in which it is administered.
Doses of 1.7mg/kg (~125mg in a ~160lb adult) have been evaluated in clinical laboratory conditions without evidence of damage to serotonin neurocircuits or functional deficits. In the six phase II trials of MDMA-AP for PTSD conducted thus far, doses have ranged between 75-187.5mg and participants have undergone 2-3 experimental sessions spaced 3-5 weeks apart. In phase III trials, the maximum dose in any session will be 120mg as an initial dose and 60mg as a booster dose (180mg total) and all participants will undergo 3 sessions. In phase II, there were no detected changes in neurocognitive function over a 2-month period following the second and third experimental sessions. During MDMA-AP sessions participants reported a range of short-lived side effects characteristic of amphetamine use and mild serotonin excess. In the week after use fatigue peaked on day 1 and decreased towards day 6, the need for more sleep peaked on day 2 and decreased towards day 6, and low mood peaked between days 2-4 and decreased towards day 6. Notably, in 4 of the 6 phase II studies, depression scores were measured and showed greater improvements than placebo over the course of the trial. In summary, it’s clear that a course of 2-3 MDMA sessions spaced at least a month apart utilizing doses of 75-125mg followed by a booster dose of 37.5mg-75mg 2-3 hours later does not produce persistent neurotoxic effects.
In the previously mentioned study of MDMA samples, the ones that contained >67% MDMA averaged 205mg per dose, higher than the initial and booster doses in MDMA-AP combined (however weights include fillers thus is likely an overestimate of MDMA per tablet). Consumption of multiple tablets (stacking) can bring doses up to 0.5g or more throughout an evening for some users; similar to amounts consumed in all 3 MDMA-AP sessions combined. Stacking was investigated in laboratory conditions by giving 100mg of MDMA followed by another 100mg of MDMA 4 hours later. Results demonstrated that blood concentrations, cardiovascular stress, and temperature were all increased, however subjective intensity of effect was similar due to development of rapid tolerance to pleasurable effects. This may cause users to underestimate the physical stress they are placing on their body with repeated dosing.
So, what does the research tell us so far?
There are two converging streams of evidence here. One from recreation literature and Ecstasy use suggesting potential for neurotoxicity. The other from clinical environments in which MDMA is predominantly therapeutic, has a promising safety profile, and lacks evidence of clinically significant neurotoxicity despite using measures that could reasonably detect such problems. Therefore, models of dosing from clinical trials could be extrapolated and generalized into a harm reduction framework that could be applied broadly to persons who use MDMA.
While phase II trials did detect several cases of persons experiencing side effects of MDMA in the week after their session, the changes were transient and resolved after a week in almost all cases. This suggests that transient changes to mood, sleep, or cognition in the week after use is a side effect that persons should be educated about, while neurotoxicity is an adverse drug reaction associated with ‘incorrect’ or harmful administration patterns.
How can MDMA-induced neurotoxicity be avoided?
The good news is that avoiding or severely limiting the potential for significant neurotoxicity occurring with MDMA is straight-forward, not difficult, and preserves the ability for profound benefits.
- Limit MDMA doses to the moderate range 75-125mg initially
- Limit dosing in a single session to a single booster of 50% the original dose
- Space MDMA sessions at least one month apart
- Limit dosing to 3-4 times per calendar year
- Avoid using other drugs and alcohol with MDMA
- Test ‘ecstasy’ tablets for presence of MDMA prior to use (as well as absence of other drugs such as fentanyl)
- Limit sleep disruption due to MDMA use
- Plan regular breaks and sip water if exposed to hot environments (do not drink excessive amounts of water)
If you notice a pattern of low mood or cognitive problems in the days after MDMA use that seems to worsen upon repeated administration or is persistent beyond a few days post-use then take an extended break from using MDMA or similar substances
Do antidepressants or 5-HTP prevent MDMA-induced neurotoxicity?
Serotonin blocking antidepressants such as Selective Serotonin Reuptake Inhibitors (SSRIs e.g. Prozac or fluoxetine) can diminish neurotoxic potential of MDMA. However, this is a poor strategy overall because serotonin blocking antidepressants also greatly diminish the subjective experience of MDMA.
Supplements such as L-tryptophan or 5-HTP are able to boost serotonin synthesis. It may not be wise to take large doses prior to MDMA use as it could increase the risk of physical side effects of serotonin excess such as nausea, vomiting, or diarrhea. Conversely, it may be reasonable to use L-tryptophan or 5-HTP for a few days after MDMA use to aid in restoring serotonin levels.
Neither antidepressants nor serotonin precursor supplementation has adequate data suggesting benefits to recommend use. While further research and testing of interventions to reduce either week after side effects or prevent neurotoxic effects is encouraged, current clinical trial data do not support such measures as necessary.
Summary & conclusions
Neurotoxicity can manifest in many forms and severity levels and MDMA appears to be neurotoxic under certain circumstances. High doses or several doses throughout the period of ingestion, frequent use (e.g. weekly use at nightlife events), aduleration, and substance mixing all are implicated in the adverse event of MDMA neurotoxicity. Data from phase II trials of MDMA-AP do not support risks of clinically significant neurotoxicity from MDMA use as side effects were observed to be mild and limited to the week after use, while participants also saw profound improvements in clinical symptoms for PTSD. In summary, MDMA induced neurotoxicity is easy to avoid with moderate doses, adequate time between use, and pure substances without combination.
In this post we’ll give some background information on what neurotoxicity is, how MDMA may act as a neurotoxin, summarize research findings on MDMA-induced neurotoxicity, and explain use parameters that are likely to prevent development of significant neurotoxic effects.

www.spiritpharmacist.com