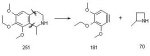
Yea, although this looks like an interesting thread botany is not really my cup of tea. I *know* this is straying off the rails but I have limited knowledge of these plant alkaloids.
I'm actually starting a an analytical position in a dairy factory tomorrow morning. If I last the full six months then I should be more of a skilled forensic chemist and not just the amateur new graduate that I am currently.
Part of the reason for my lack of motivation is the lack of flexibility of the laws in the uk. As people in this forum know my interests lie in development of 'Arecaine', 'Turricaine' (aka 'Nuclear Knockout' or 'Parkaine') and allied molecules such as Green and Blue Nickel [see my gallery for clarrification]. Also an enantioselctive version is on the cards if I can reach the stage where I am able to convince industry and possible surpase current drugs such as Sertraline and Paxil.
If I can develop the synthetic methodology up to an acceptable level I am planning on going into the patenting phase of the project sometime in the next few hundred days using the money I collect from doing the 'milk rounds' to drive the reactants over into something tangable.
However, I am not so self-centered as to think that other people dont have projects of their own that are worth pursuing. I suppose my real interests lie in bringing molecules back from the field trip and sitting down with an open mind planning on how to make tese molecules better or in some way more suitable for a commercial audience.
The 3,4-methlenedioxyl-isoquinoline was an example of taking an in-vivo process in-vitro. However, whilst 3,4-methlyenedioxyPEA is readily synthesizable I accept that this is highly illegal in the UK. In the US though a skilled chemist could just about do this legitametely since it not really an anlalog of MDA given that the product is a precursor and will be inactive on its own.
The question remains elusive as to its bioactivity data (if it is active then at what dosage). I know this post is mostly unrelated to your threasd but I was just trying to inject some enthusaism into an area that interests me that I know nothing about. I have no interest in halucinogens but entactogens DO interest me.
I'm actually starting a an analytical position in a dairy factory tomorrow morning. If I last the full six months then I should be more of a skilled forensic chemist and not just the amateur new graduate that I am currently.
Part of the reason for my lack of motivation is the lack of flexibility of the laws in the uk. As people in this forum know my interests lie in development of 'Arecaine', 'Turricaine' (aka 'Nuclear Knockout' or 'Parkaine') and allied molecules such as Green and Blue Nickel [see my gallery for clarrification]. Also an enantioselctive version is on the cards if I can reach the stage where I am able to convince industry and possible surpase current drugs such as Sertraline and Paxil.
If I can develop the synthetic methodology up to an acceptable level I am planning on going into the patenting phase of the project sometime in the next few hundred days using the money I collect from doing the 'milk rounds' to drive the reactants over into something tangable.
However, I am not so self-centered as to think that other people dont have projects of their own that are worth pursuing. I suppose my real interests lie in bringing molecules back from the field trip and sitting down with an open mind planning on how to make tese molecules better or in some way more suitable for a commercial audience.
The 3,4-methlenedioxyl-isoquinoline was an example of taking an in-vivo process in-vitro. However, whilst 3,4-methlyenedioxyPEA is readily synthesizable I accept that this is highly illegal in the UK. In the US though a skilled chemist could just about do this legitametely since it not really an anlalog of MDA given that the product is a precursor and will be inactive on its own.
The question remains elusive as to its bioactivity data (if it is active then at what dosage). I know this post is mostly unrelated to your threasd but I was just trying to inject some enthusaism into an area that interests me that I know nothing about. I have no interest in halucinogens but entactogens DO interest me.