Grignard said:
Hmm, there's nothing in Beilstein about it? Can you get any NMR spectra on your unknown? Also I dunno if you have the ability or the authority, but can you synthesize an authentic standard and compare it against this?
Yes, Yes...C13 NMR would be the way to go, but I would still have to sort through an intepretation, and a synthesis would be a lot more work. Just having some reference spectra of tetrahydroisoquinolines from the literature would be nice.
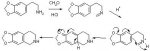
In the meantime, I'm left to ponder the interesting similarity between the major component's mass spec shown above and this minor component's mass spec:
Note difference of 14 m/z units for the M+ ion (251-14=237) and the most abundant fragment (70-14=56) , yet they share a common fragment of 181 mass units.
This appears to be a compound related to the major component but replaces a hydrogen with the a methyl group, i.e. a net change of 14 units.
Keeping in mind my proposed "retro-Diels-Alder fragmentation" pattern, I would assume the 181 fragment appears from the trimethyoxy isoquinoline structure and the difference occurs on the right-hand side of the molecule....that is, the minor component is the O-methyl derivative of either of these two compounds:
While the unknown in question is related to the major component O-methylpellotine (MW 251) by having a hydrogen substitute for one of the methyl groups (MW 251-14=237), it is
NOT pellotine,
For it were, it would NOT produce the 181 m/z ion indicative of a trimethoxy substituted compound.
Having a reference mass spec of any of these isoquinolines would be nice to have for comparison....I haven't been able to find one yet