phase_dancer
Bluelight Crew
Wicked Posts Biscuit and fairnymph!
Biscuit you are correct in saying the d (+) form of pseudo is probably what you find in OTC preparations, unless its synthetic in which case it could well be the dl form (i.e. racemic).
If meth is made from naturally occurring pseudo or ephedrine i.e. it was originally extracted from Ma Huang, ephedra etc., ALL the reduction product will be the d isomer.
My old Pharm bible by Goodman and Gillman’s [1980] lists preparations containing only the l form of ephedrine. Things could be different toady, but I doubt it.
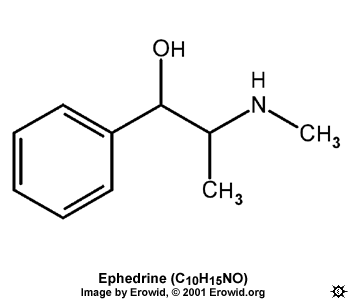
If you look at molecules of pseudo and ephedrine, ( naturally occurring l-eph and d-pseudo ) you will notice the only difference is where the H and the OH are positioned on the 1st carbon of the alkyl chain. These isomers are known as diastereomers – not to be confused with enantiomers (l + d ) These will not produce different products (in this case anyway). This means that any process which does something to the OH on natural ephedrine will do the same to OH on natural pseudo.
FROM RHODIUM
d-Ephedrine has not been found naturally. The synthetic base has mp 40-40.5°C; the hydrochloride is in the form of white leaflets, mp 216-217°C.
dl-Ephedrine (racemic ephedrine), is the synthetic, inactive ephedrine of commerce. The free base has mp 76-78°C; hydrochloride, mp 187-188°C.
l-pseudo-Ephedrine has not been found in nature. The base has mp 118-118.7°C. The hydrochloride has mp 182-182.5°C. The l-pseudo-ephedrine-d-tartrate has mp 178°C; l-pseudo-ephedrine-l-tartrate, mp 178.5°C (17, 68, 69, 70).
dl-pseudo-Ephedrine (racemic pseudo-ephedrine) melts at 118°C. The hydrochloride has a mp of 164°C.
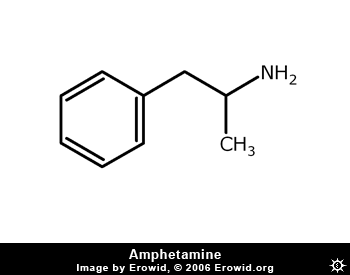
Not all chemicals exist as enantiomers. It depends upon whether a chiral carbon exists. (4 different groups attached to 1 carbon). And there are many other types of isomers. Ephedrine has 2 chiral centers and Amphetamine has 1. So for ephedrine there exist 4 possible optical isomers. Looking at amphetamine, one may say there are two – a 2nd with the nitrogen - but as the amine does not form a tetrahedral shape, it does not affect the optical conformation of the molecule.
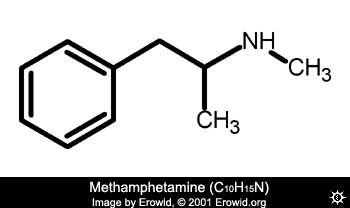
Stories abound regarding whacky procedures for manufacturing meth. Check this out for myth clarification. Hope’s it still OK to post appropriate pages from this site.
”An investigation of the extraction of methamphetamine from chicken feed, and other myths”
http://rhodium.ws/chemistry/chickenfeed.html
Biscuit you are correct in saying the d (+) form of pseudo is probably what you find in OTC preparations, unless its synthetic in which case it could well be the dl form (i.e. racemic).
If meth is made from naturally occurring pseudo or ephedrine i.e. it was originally extracted from Ma Huang, ephedra etc., ALL the reduction product will be the d isomer.
My old Pharm bible by Goodman and Gillman’s [1980] lists preparations containing only the l form of ephedrine. Things could be different toady, but I doubt it.
If you look at molecules of pseudo and ephedrine, ( naturally occurring l-eph and d-pseudo ) you will notice the only difference is where the H and the OH are positioned on the 1st carbon of the alkyl chain. These isomers are known as diastereomers – not to be confused with enantiomers (l + d ) These will not produce different products (in this case anyway). This means that any process which does something to the OH on natural ephedrine will do the same to OH on natural pseudo.
FROM RHODIUM
d-Ephedrine has not been found naturally. The synthetic base has mp 40-40.5°C; the hydrochloride is in the form of white leaflets, mp 216-217°C.
dl-Ephedrine (racemic ephedrine), is the synthetic, inactive ephedrine of commerce. The free base has mp 76-78°C; hydrochloride, mp 187-188°C.
l-pseudo-Ephedrine has not been found in nature. The base has mp 118-118.7°C. The hydrochloride has mp 182-182.5°C. The l-pseudo-ephedrine-d-tartrate has mp 178°C; l-pseudo-ephedrine-l-tartrate, mp 178.5°C (17, 68, 69, 70).
dl-pseudo-Ephedrine (racemic pseudo-ephedrine) melts at 118°C. The hydrochloride has a mp of 164°C.
Not all chemicals exist as enantiomers. It depends upon whether a chiral carbon exists. (4 different groups attached to 1 carbon). And there are many other types of isomers. Ephedrine has 2 chiral centers and Amphetamine has 1. So for ephedrine there exist 4 possible optical isomers. Looking at amphetamine, one may say there are two – a 2nd with the nitrogen - but as the amine does not form a tetrahedral shape, it does not affect the optical conformation of the molecule.
Stories abound regarding whacky procedures for manufacturing meth. Check this out for myth clarification. Hope’s it still OK to post appropriate pages from this site.
”An investigation of the extraction of methamphetamine from chicken feed, and other myths”
http://rhodium.ws/chemistry/chickenfeed.html