fastandbulbous
Bluelight Crew
Phenolic OH groups are way more polar than plain hydrocarbon OH groups (it's why phenol's old name is carbolic acid - it forms salts with sodium ions!). Morphine does pass the blood brain barrier, but esterification of the phenolic OH group allows it to enter so much easier (you may have heard of such a thing, diacetylmorphine aka heroin/smack). Now, esterify that 4 hydroxy phenolic group and it will pass through the bbb so much easier. Unfortunately, it won't help that much, as the oxygen at the 3 position needs to be held rigidly in position, so it's lone pairs can interact with assorted receptors (why 6-APB is still a potent entactogen, but 3,4-dimethoxyamphetamine is well, shite in comparisonHuman Blood Brain Barrier Basics: A hydrocarbon (HC) can have up to one carboxylic acid group (R-CO2H) and up to three alcoholic (R-OH) functional groups. However, even one phenolic hydroxyl group appears to be a no go. If I'm wrong about that last point, I would love to be corrected. It is based on the solitairy fact that 2-methylamino-eugenol is approximately 1/50th the potency of MDMA (aka 2-methylaminosafrole).
EDIT: Morphine gets in the brain and has 1 aliphatic alcohol and 1 phenolic alcohol, so perhaps my eugenolic methamphetamine info is spurious; is it active after all?
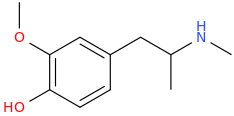
EUGENIUS
1-(3-methoxy-4-hydroxyphenyl)-2-methylaminopropane
I do know that eugenius is a metabolite of MDMA.



