fastandbulbous
Bluelight Crew
The plant kratom (M. speciosa) has been used in its native Thailand to treat opium addiction, and alkaloids found in the plant have been discovered to have mu opiate receptor agonist activity.
Some people find it difficult to believe that an indole/tryptamine alkaloid, with a close relationship to psychedelic tryptamines and yohimbine could possess opiate activity, but the alkaloids found conform to the basic chemical structure requirement needed for mu agonist activity

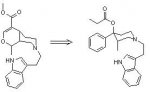
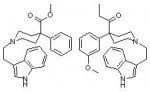
The main alkaloid, mitragynine has some mu and delta opiate agonism, but the most active agonists are the alkaloids which have the pseudoxindole structure. Shown below are the structures of mitragynine and the correspondind pseudoxindole, with the basic opiate requirement skeleton shown in red

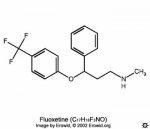
As can be seen, both consist of a phenyl ring separated from a tertiary amine by 3 carbon atoms. Mitragynine does not fully match the structure, as the carbon atom (noted by *) attached to the phenyl group needs to be fully substituted for maximun agonist activity, and most probably accounts for why the pseudoxindole is the most active compound structure (and is closest to the required bond angles due to sp3 hybridization).
Quite why these alkaloids have opiate activity, but other similar ones, such as yohimbine, do not is a bit of a mystery to me at the minute, but any further contributions/insights would be greatly appreciated
Some people find it difficult to believe that an indole/tryptamine alkaloid, with a close relationship to psychedelic tryptamines and yohimbine could possess opiate activity, but the alkaloids found conform to the basic chemical structure requirement needed for mu agonist activity
The main alkaloid, mitragynine has some mu and delta opiate agonism, but the most active agonists are the alkaloids which have the pseudoxindole structure. Shown below are the structures of mitragynine and the correspondind pseudoxindole, with the basic opiate requirement skeleton shown in red
As can be seen, both consist of a phenyl ring separated from a tertiary amine by 3 carbon atoms. Mitragynine does not fully match the structure, as the carbon atom (noted by *) attached to the phenyl group needs to be fully substituted for maximun agonist activity, and most probably accounts for why the pseudoxindole is the most active compound structure (and is closest to the required bond angles due to sp3 hybridization).
Quite why these alkaloids have opiate activity, but other similar ones, such as yohimbine, do not is a bit of a mystery to me at the minute, but any further contributions/insights would be greatly appreciated