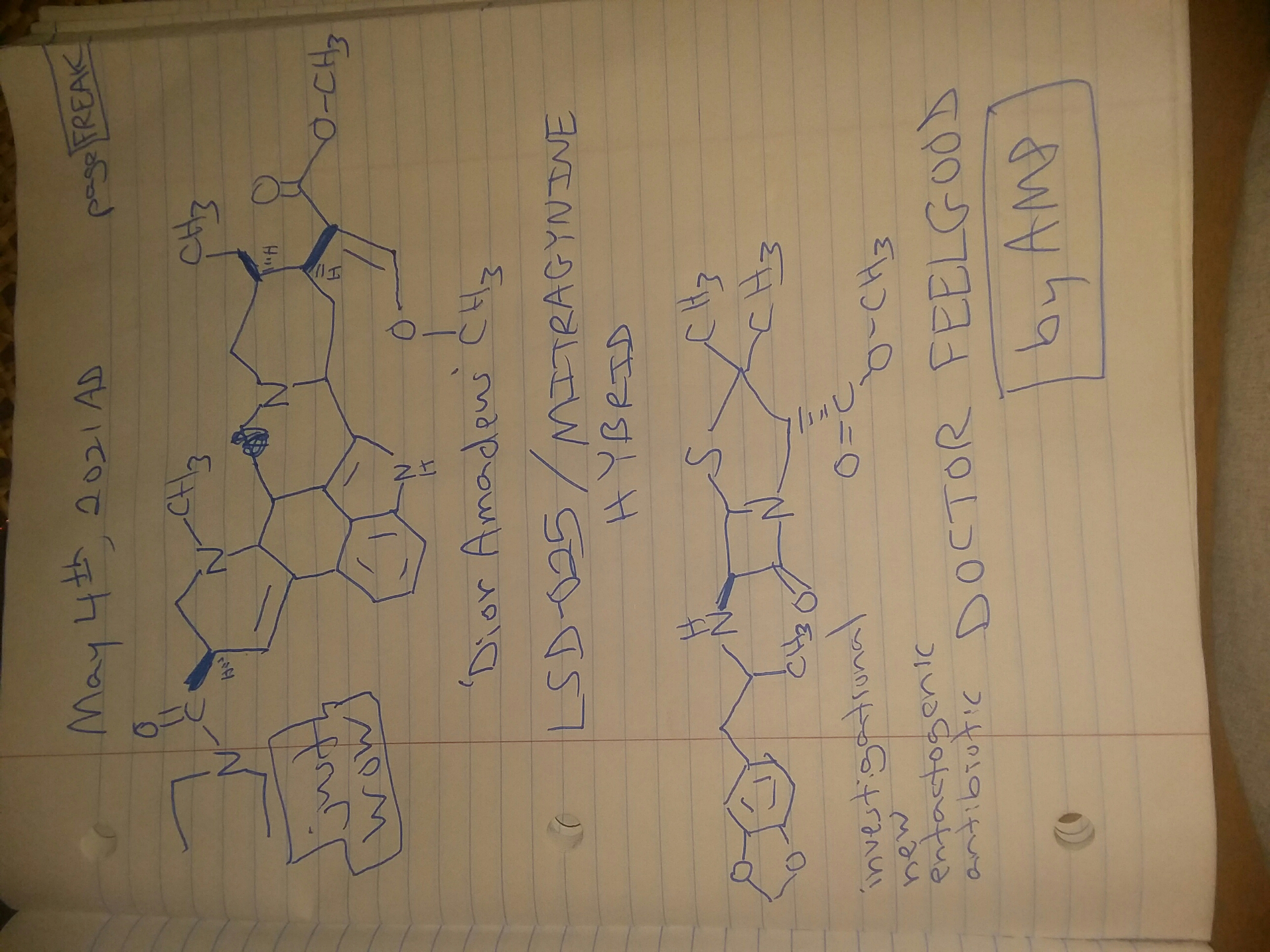
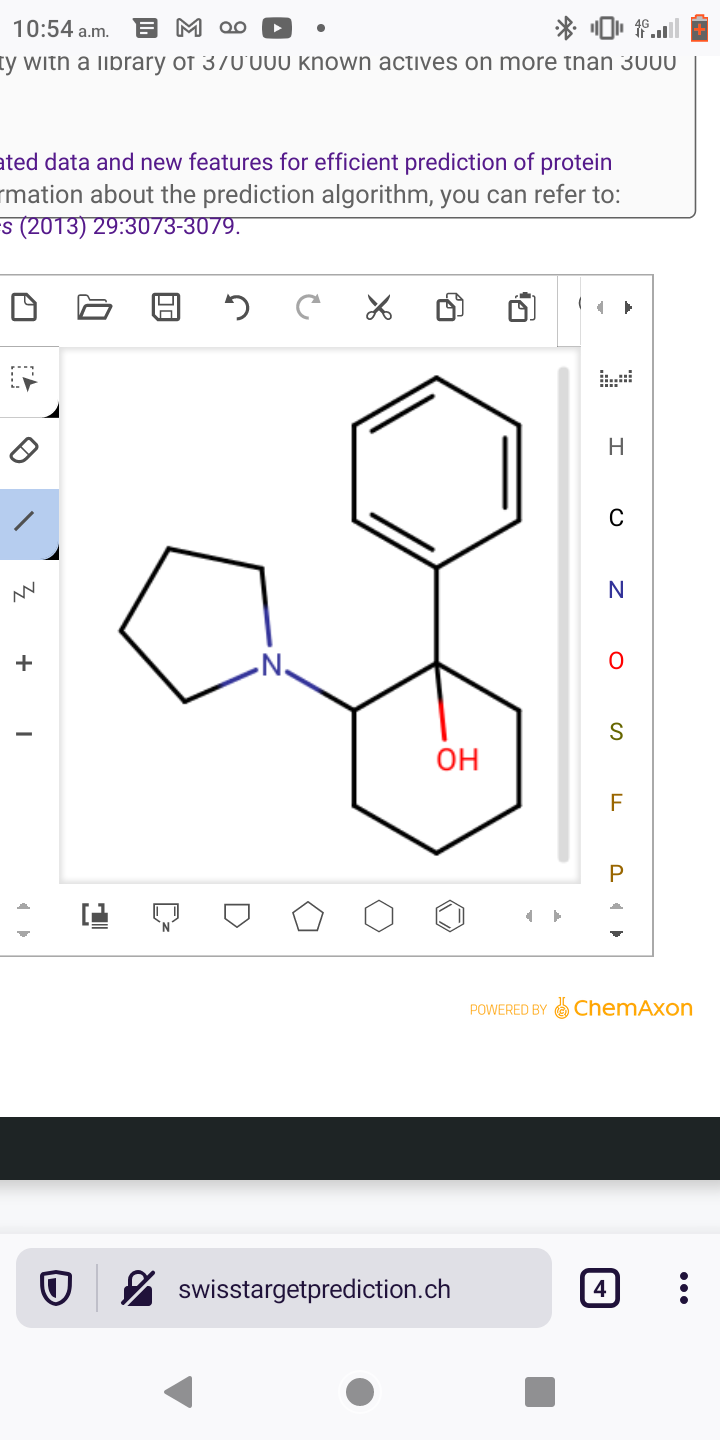
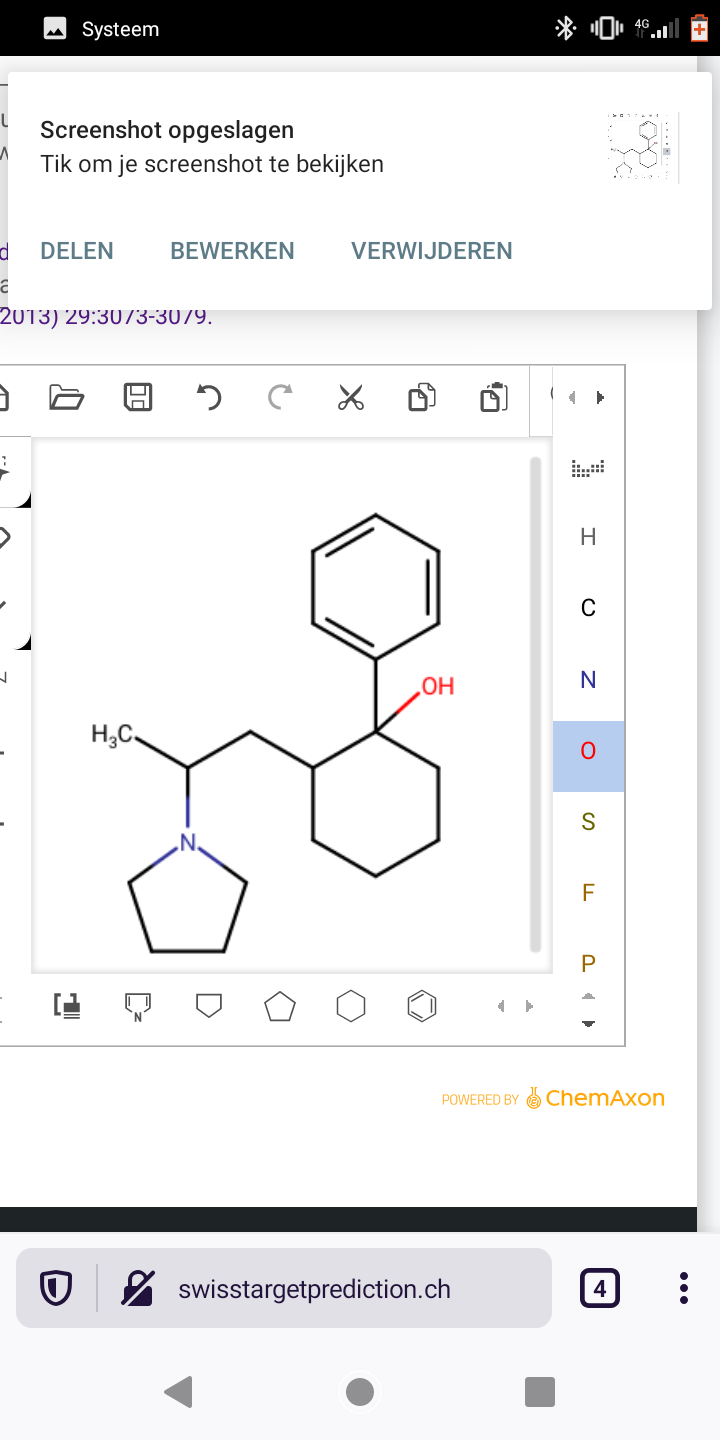
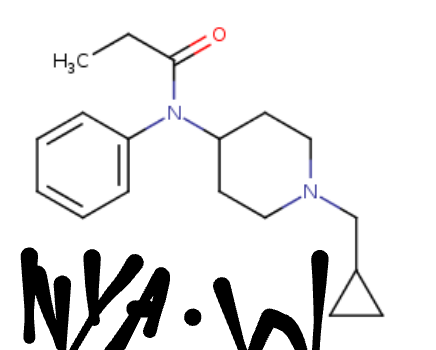
Abstract
Novel synthetic compounds have been available for decades as quasi-legal alternatives to controlled substances. The hallucinogen-like effects of eight novel substituted tryptamines were evaluated to determine their potential abuse liability. Male Sprague-Dawley rats were trained to discriminate 2,5-dimethoxy-4-methylamphetamine (DOM, 0.5 mg/kg, i.p., 30 min) from saline. 4-Acetoxy-N,N-diethyltryptamine (4-AcO-DET), 4-hydroxy-N-methyl-N-ethyltryptamine (4-OH-MET), 4-hydroxy-N,N-diethyltryptamine (4-OH-DET), 4-acetoxy-N-methyl-N-isopropyltryptamine (4-AcO-MiPT), 4-acetoxy-N,N-dimethyltryptamine (4-AcO-DMT), 4-hydroxy-N,N-dimethyltryptamine (4-OH-DMT, psilocin), 5-methoxy-N-methyl-N-isopropyltryptamine (5-MeO-MiPT), 4-acetoxy-N,N-diisopropyltryptamine (4-AcO-DiPT), and 4-hydroxy-N,N-diisopropyltryptamine (4-OH-DiPT) were tested for their ability to substitute for the discriminative stimulus effects of DOM. All test compounds fully substituted for DOM with potencies less than or equal to that of DOM. 4-OH-MET, 4-OH-DET, 4-OH-DMT, and 4-AcO-DMT decreased response rate at doses that fully substituted. Because the test compounds produced DOM-like discriminative stimulus effects, they may have similar abuse liability as DOM. 4-Acetoxy substituted compounds were less potent than 4-hydroxy substituted compounds, and the N,N-diisopropyl compounds were less potent than the dimethyl, diethyl, N-methyl-N-ethyl, and N-methyl-N-isopropyl compounds.





![2-ethylamino-2-(3-methoxyphenyl)-bicyclo[2.2.2]octane.png](/community/proxy.php?image=https%3A%2F%2Fopsin.ch.cam.ac.uk%2Fopsin%2F2-ethylamino-2-%283-methoxyphenyl%29-bicyclo%5B2.2.2%5Doctane.png&hash=0abf7e1a04a4cedefc3112b7845b8dcf)
![1-(3-pyridinyl)-N-methyl-2-azabicyclo[2.2.1]heptane.png](/community/proxy.php?image=https%3A%2F%2Fopsin.ch.cam.ac.uk%2Fopsin%2F1-%283-pyridinyl%29-N-methyl-2-azabicyclo%5B2.2.1%5Dheptane.png&hash=7310037133d6c00479b82fd261a28ffe)
![N-methyl-5-phenyl-3-azabicyclo[2.2.1]heptane.png](/community/proxy.php?image=https%3A%2F%2Fopsin.ch.cam.ac.uk%2Fopsin%2FN-methyl-5-phenyl-3-azabicyclo%5B2.2.1%5Dheptane.png&hash=7434c83a81055bc02e04fa9f332444cf)
![N-ethyl-5-(3,4-methylenedioxyphenyloxy)-3-azabicyclo[2.2.1]heptane.png](/community/proxy.php?image=https%3A%2F%2Fopsin.ch.cam.ac.uk%2Fopsin%2FN-ethyl-5-%283%2C4-methylenedioxyphenyloxy%29-3-azabicyclo%5B2.2.1%5Dheptane.png&hash=bdd843e2dc7656526056d54d37297a6c)
![N-methyl-5-phenyloxy-3-azabicyclo[2.2.1]heptane.png](/community/proxy.php?image=https%3A%2F%2Fopsin.ch.cam.ac.uk%2Fopsin%2FN-methyl-5-phenyloxy-3-azabicyclo%5B2.2.1%5Dheptane.png&hash=d015bc19b025200305983fe244e44604)
![1-(2-azabicyclo[2.2.2]octane-1-yl)-1-carbomethoxy-1-phenylmethane.png](/community/proxy.php?image=https%3A%2F%2Fopsin.ch.cam.ac.uk%2Fopsin%2F1-%282-azabicyclo%5B2.2.2%5Doctane-1-yl%29-1-carbomethoxy-1-phenylmethane.png&hash=abae7518f83a9778ea42928acecb435c)
![1-(2-azabicyclo[2.2.2]octane-3-yl)-1-carbomethoxy-1-phenylmethane.png](/community/proxy.php?image=https%3A%2F%2Fopsin.ch.cam.ac.uk%2Fopsin%2F1-%282-azabicyclo%5B2.2.2%5Doctane-3-yl%29-1-carbomethoxy-1-phenylmethane.png&hash=f5700df58cd6bcf718a2508a34fb81fa)
![1-(2-azabicyclo[2.2.2]octane-3-yl)-1-carbomethoxy-1-(3,4-methylenedioxyphenyl)methane.png](/community/proxy.php?image=https%3A%2F%2Fopsin.ch.cam.ac.uk%2Fopsin%2F1-%282-azabicyclo%5B2.2.2%5Doctane-3-yl%29-1-carbomethoxy-1-%283%2C4-methylenedioxyphenyl%29methane.png&hash=7754477dbd4cf5dbae81a5065022fd49)
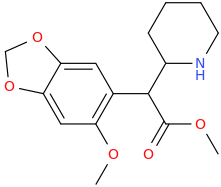
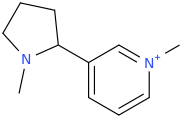
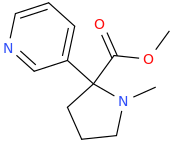
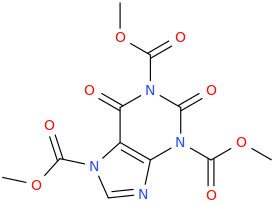
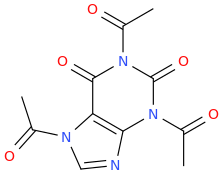




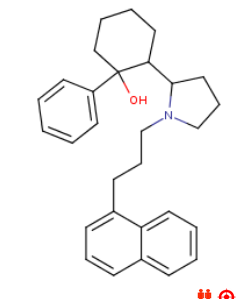
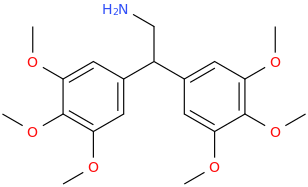
![6-(3-(1-carbomethoxy-1-phenylmethyl)-2-azacyclohexyl)-3-propylthio-2-carbomethoxy-7-oxo-(azabicyclo[3.2.0]hept-2-ene).png](/community/proxy.php?image=https%3A%2F%2Fopsin.ch.cam.ac.uk%2Fopsin%2F6-%283-%281-carbomethoxy-1-phenylmethyl%29-2-azacyclohexyl%29-3-propylthio-2-carbomethoxy-7-oxo-%28azabicyclo%5B3.2.0%5Dhept-2-ene%29.png&hash=dc1ec7786194e2c04ed800e36031f503)
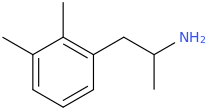
![(2S,6S)-6-(1-aza-2-methyl-3-phenylpropyl)-3-propylthio-2-carbomethoxy-7-oxo-(azabicyclo[3.2.0]hept-2-ene).png](/community/proxy.php?image=https%3A%2F%2Fopsin.ch.cam.ac.uk%2Fopsin%2F%282S%2C6S%29-6-%281-aza-2-methyl-3-phenylpropyl%29-3-propylthio-2-carbomethoxy-7-oxo-%28azabicyclo%5B3.2.0%5Dhept-2-ene%29.png&hash=94915ed806c88d4b30bf8b4767d55736)