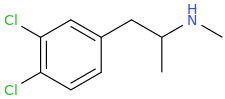
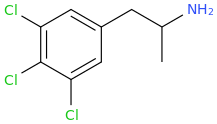
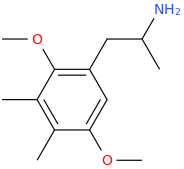
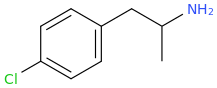
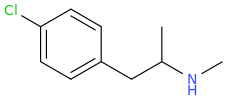
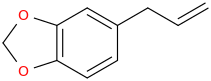
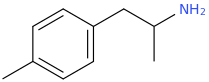
^^Interesting would this work? What might the effects be?
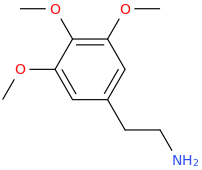
The effects of the top structure, *may* be thought to be similar to mescaline, which has a similar structure; that is the hope anyway.
MESCALINE
However, a published scientific study I read once, found this similar drug only to be a stimulant, not a psychedelic.
^--what the published study was about.
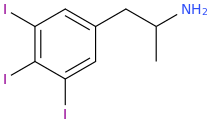
So,1-(3,4,5-trichlorophenyl)-2-aminopropane (SHIVA, to complement the GANESHA of PiHKAL notoriety) might also only be expected to be a stimulant as well; however, I hope it is much more than that.
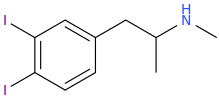
As for the other one,
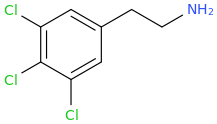
it could, according to the published scientific literature to date, reasonably be safe to assume as a MDMA like emphathogenic / stimulant combo drug. Am I right about this last point, science guys? I know that 4-chloroamphetamine can pass for very good, high-dose MDMA, based on a user trip report of Diablo XXX Party Pills, which were known to contain PCA.
SHIVA (my naming)
GANESHA (Shulgin's naming)
(actually, to be rigorously forthcoming it is unclear from reading PiHKAL by Sasha Shulgin whether his invention, GANESHA, was the ethane [PEA] or propane [amphetamine] form of the drug, but according to what else he said there about the series, it can reasonably be assumed that the PEA form of this drug is equally or very nearly equally as potent as the amphetamine version).
PCA
Whether all these new drugs have a chloro or an iodo should only make for minor qualitative differences between and among these drugs; thus, you asked about the iodo forms, but I answered using the chloro forms. They are practically interchangeable, as halogens, as far as answering this question is concerned anyway.
Finally, to possibly further indemnify the belief in anyone that this post may be at least somewhat germane to the comings and goings of the black market, street drug world which most of us BL users find ourselves subservient to in that we depend on its wares as often as 1x per day, on March of this year, 2016, in Vienna, Austria, a pressed pill was found to contain this ring halogenated methamphetamine, according to ecstasydata.org:
PCMA
And yet, no apparent PMA/PMMA type overdose deaths among ecstasy pill poppers was noted there recently, to my knowledge. A good thing about the halogenated amphetamines, other than apparently qualitatively substituting for MDMA, is that their synthesis does not depend completely on the obtainment of the very hard to get, very watched, very hard to make from scratch,
SAFROLE
the heavenly smelling chemical whose absence of which made widespread, available street MDMA an impossibility for a good decade in the early 2000s and still does in everywhere in the world but the clandestine labs of Europe, whose chemists have discovered another, commercially available and unnamed by me precusor for their Teslas, Dominos, Bugattis, and whatnot high dosed pressed MDMA pills.