Morninggloryseed
Bluelight Crew
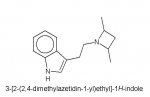
I’m very surprised that I’ve not seen this anywhere. Since the “azetidine” analogue of LSD is known, and of extreme potency…why has no one thought to incoperate this into a tryptamine structure? I know of other IEA’s that end in rings and retain activity. Since this worked on LSD (where usually any change abolishes activity), I see no reason for it not to work here. It is easy to see DiPT, EIPT, and other N,N-dialkyltryptamines in this new structure. “Azetitryptamine”
What do you brains think of this one?
What do you brains think of this one?