-
Neuroscience & Pharmacology Discussion Welcome Guest
Posting Rules Bluelight Rules Recent Journal Articles Chemistry Mega-Thread FREE Chemistry Databases! Self-Education Guide -
N&PD Moderators: Skorpio | someguyontheinternet
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Ibogamine A better alternative to ibogaine for those who cannot tolerate or dislike
- Thread starter Neuroprotection
- Start date
Ibogaine doesn't sound to be dissociative? All the reports of flood doses I read sound like deep dissociative state and wandering around your mind, actually sounds a lot like Salvia Divinorum experience but much much longer lasting. It could be a result of both dissociative effect caused via NMDA receptors and/or sigma receptors and serotonin reuptake inhibition. I experienced similar states after DXM though they were mostly abstract at moderate doses rather than making up long stories that you take part in. Certain arylcyclohexanamines produce similar effects too. Still, that's what dissociation is in my experience, losing contact with your environment and experiencing deep continuous introspection however it is effected.
Totally agree. Ibogaine and its main metabolite norIbogaine effect is very much similar to that of DMX and its major metabolite Dextrorphan. Salvia is much more selective (pure kappa which I believe is the basis of Iogaine "hallucinations"). But the overall profile of IBG is very close to that of DMX. Check out their binding profile. DMX is just a less potent (10-100x) IBG across the board (cant find data for SERT/NET Ki for IBG. please post if by any chance you came across). So dosing DMX might as well give the same effect as IBG. Or even better norDMX.(as far as kappa is concerned: it is 1280 times more potent than the methoxylated DMX!

Incidentally both Ibogaine and DMX are metabolized by demethylation (18methoxy ---> 18-hydroxy in IBG and 5-methoxy-->5-hydroxy in DMX/DXO. Probably this may have something to do with the antiaddiction/hallucinations of Ibogaine and DMX. In both case, the 18-hydroxy is a much more potent Kappa agonist in both case.
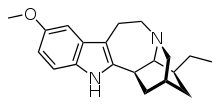
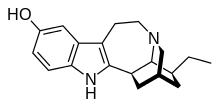
and DMX/DXO
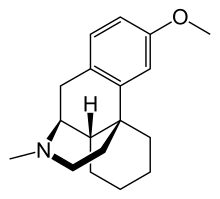
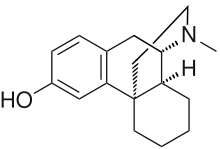
nb: timing of norIbogaine metabolites formation after oral Iboga ingestion corresponds roughly to the comeup of the so-called psych introspection phase of IBG trip. Definitely different from 5HT2A trip. Don't know how to describe that not really hallucinations of the type 2A agonist. very much like prolong Salvia trip.
Question is: Can Salvia trip(s) provides same antiaddictve effect as Ibogaine? Or mega-doses of DMX for that matter? (give me BL nobel prize if anybody finds out!!)
Last edited:
serotonin2A
Bluelighter
- Joined
- Sep 13, 2014
- Messages
- 1,354
^I think DotChem's post on the pharmacology is pretty convincing that the kappa and NMDA-R effects of ibogaine are not solely responsible for the antiaddictive effects. Howard Lotsof discovered those properties of ibogaine after he tried it, and he lost all desire to continue to use heroin. That type of effect is completely different from what dextromethorphan or salvinorin A do. People aren't taking the latter drugs and deciding to walk away from heroin addictions. NMDA-R antagonists and KOR agonists certainly have utility against tolerance and addiction, but not in the same way that ibogaine does.
Lotsof always hypothesized that ibogaine was reprogramming the limbic system, and the nicotinic effects of ibogaine may actually be able to tap into such a mechanism. The alpha3beta4 nicotinic receptors are primarly expressed in the habenula, which is one of the main sources of excitatory glutamatergic input to the ventral tegmental area (VTA). The VTA, of course, contains the dopaminergic neurons responsible for the reinforcing effects of cocaine, heroin, alcohol, and opiates. It is possible that altering excitatory input into the VTA can counteract the habituated patterns of motor behavior and the sensitization that make it extremely difficult to walk away from addiction. The other psychoactive effects of ibogaine (NMDA-R blockade, KOR and MOR activation, 5-HT2A) may complement the nicotinic effects by relieving withdrawal symptoms, and possibly by producing therapeutic mystical-type experiences. Those effects would help instill the motivation to stop using drugs, and the ability to walk away from addiction without experiencing withdrawal. But it really does seem like all those other effects are not themselves sufficient to produce ibogaine-like effects on addiction.
Lotsof always hypothesized that ibogaine was reprogramming the limbic system, and the nicotinic effects of ibogaine may actually be able to tap into such a mechanism. The alpha3beta4 nicotinic receptors are primarly expressed in the habenula, which is one of the main sources of excitatory glutamatergic input to the ventral tegmental area (VTA). The VTA, of course, contains the dopaminergic neurons responsible for the reinforcing effects of cocaine, heroin, alcohol, and opiates. It is possible that altering excitatory input into the VTA can counteract the habituated patterns of motor behavior and the sensitization that make it extremely difficult to walk away from addiction. The other psychoactive effects of ibogaine (NMDA-R blockade, KOR and MOR activation, 5-HT2A) may complement the nicotinic effects by relieving withdrawal symptoms, and possibly by producing therapeutic mystical-type experiences. Those effects would help instill the motivation to stop using drugs, and the ability to walk away from addiction without experiencing withdrawal. But it really does seem like all those other effects are not themselves sufficient to produce ibogaine-like effects on addiction.
Are there other a3b4 nicotinic agents known that could be compared? I mean, if the topic of the thread and, of course, the aim of pretty much all drug design, is to isolate the anti-addictive effect... it would be pretty interesting to see what selective agonists (? EDIT: so no antagonists then instead) would do. Also make a group in the study combine it with dizolcipine and 25I-NBOMe and idk a little bit of the right opioid, to simulate what may be the complementary effects. Although, I would say it might not be strictly necessary as the therapeutic potential of psychedelics and NMDA antagonists on their own have already kind of shown that it would be hard to dismiss as a factor.
Very tentative would be to instead use neurotrophic factor affecting drugs like the supposed 'adaptogens' selank and semaks, to compound 'rewiring' effects.
A trip can be an important factor when positive 'brainwashing' is concerned, since it seems like it can catalyze or promote mental processes... now I know that we don't really know enough about nootropics especially the novel ones, but if they turn out as promised, it could be interesting to see if anti-addictive effects are catalyzed by them.
Very tentative would be to instead use neurotrophic factor affecting drugs like the supposed 'adaptogens' selank and semaks, to compound 'rewiring' effects.
A trip can be an important factor when positive 'brainwashing' is concerned, since it seems like it can catalyze or promote mental processes... now I know that we don't really know enough about nootropics especially the novel ones, but if they turn out as promised, it could be interesting to see if anti-addictive effects are catalyzed by them.
Last edited:
Cotcha Yankinov
Bluelight Crew
- Joined
- Jul 21, 2015
- Messages
- 2,952
Are there other a3b4 nicotinic agents known that could be compared?.
http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4358631/ Here is a potent and selective a3b4 antagonist that is based of the alpha conotoxins (that I'm interested in because selective a3b4 antagonists have proven effective for neuropathic pain, and idk about taking ibogaine every single day lol)
That is what it sounds like to me, but I don't know if I am understanding it correctly.
In any case, telling people that they are using carnivorous marine snail extracts to wipe their addiction seems likely to produce a very nice placebo effect at the very least.
In any case, telling people that they are using carnivorous marine snail extracts to wipe their addiction seems likely to produce a very nice placebo effect at the very least.
serotonin2A
Bluelighter
- Joined
- Sep 13, 2014
- Messages
- 1,354
Are there other a3b4 nicotinic agents known that could be compared?
Compared with ibogaine, 18-methoxycoronaridine is much more selective for alpha3beta4. But 18-MC still has uM affinity for KOR and MOR:
http://www.ncbi.nlm.nih.gov/pubmed/11906717
http://www.ncbi.nlm.nih.gov/pubmed/15862802
http://www.ncbi.nlm.nih.gov/pubmed/8782860
http://www.ncbi.nlm.nih.gov/pubmed/10793182
http://www.ncbi.nlm.nih.gov/pubmed/10884062
http://www.ncbi.nlm.nih.gov/pubmed/11085336
http://www.ncbi.nlm.nih.gov/pubmed/16626688
http://www.ncbi.nlm.nih.gov/pubmed/17447255
Last edited:
Cotcha Yankinov
Bluelight Crew
- Joined
- Jul 21, 2015
- Messages
- 2,952
Does a little blockade of a3b4 day by day function the same as a massive blockade akin to an ibogaine flood dose?
Last edited:
serotonin2A
Bluelighter
- Joined
- Sep 13, 2014
- Messages
- 1,354
My gut feeling is that it probably wouldn't. Apparenty dextromethorphan, bupropion, and mecamylamine can also bind to the same nicotinic receptor to some extent, but they don't really mimic the effects of ibogaine. However, Glick was able to produce an anti-addictive effect by combining low doses of a few different ligands. That makes me think that there have to be pretty high levels of blockade.Does a little blockade of a3b4 day by day function the same as a massive blockade akin to an ibogaine flood dose?
On the other hand, there might be differences between how iboga alkaloids bind to the nicotinic receptor vs other ligands that effectively make the kinetics of blockade very different.
Last edited:
Cotcha Yankinov
Bluelight Crew
- Joined
- Jul 21, 2015
- Messages
- 2,952
I recall a study showing rodents can display increased locomotion up to one year after a single amphetamine dose. Do you think a3b4 antagonists could have utility for former addicts who are currently abstaining but still having issues with hyper-excitability?
Is it possible that addicting drugs and behaviors stimulate a3b4, and thus a3b4 blockade is not just a means to an end that is unrelated to the original cause?
I managed to find this study http://www.ncbi.nlm.nih.gov/m/pubmed/24750073/ regarding genetic models but it seems hard to interpret because you would think that even modifying the basal level of a3b4 input to the VTA (or knocking it out) would change the dynamics of the VTA (and therefore withdrawal) regardless of whether the drugs were stimulating a3b4 (I hope that makes sense). In other words, are drugs/behaviors actually affecting the a3b4, or is the a3b4 this somewhat "static" input that doesn't get affected too much by the surrounding brain activity, but yet blockade of a3b4 can lead to anti-addictive effects?
Do we have any idea what's providing input to or increasing the excitability of these a3b4 receptors? (Maybe that's another therapeutic target with different subtleties).
Regarding the variable mechanics of nicotinic blockade, is this similar to the concept of a slow dissossociating NMDA antagonist vs. something like memantine, rather than the concept of something like a biased ligand?
Is it possible that addicting drugs and behaviors stimulate a3b4, and thus a3b4 blockade is not just a means to an end that is unrelated to the original cause?
I managed to find this study http://www.ncbi.nlm.nih.gov/m/pubmed/24750073/ regarding genetic models but it seems hard to interpret because you would think that even modifying the basal level of a3b4 input to the VTA (or knocking it out) would change the dynamics of the VTA (and therefore withdrawal) regardless of whether the drugs were stimulating a3b4 (I hope that makes sense). In other words, are drugs/behaviors actually affecting the a3b4, or is the a3b4 this somewhat "static" input that doesn't get affected too much by the surrounding brain activity, but yet blockade of a3b4 can lead to anti-addictive effects?
Do we have any idea what's providing input to or increasing the excitability of these a3b4 receptors? (Maybe that's another therapeutic target with different subtleties).
Regarding the variable mechanics of nicotinic blockade, is this similar to the concept of a slow dissossociating NMDA antagonist vs. something like memantine, rather than the concept of something like a biased ligand?
Last edited:
serotonin2A
Bluelighter
- Joined
- Sep 13, 2014
- Messages
- 1,354
I recall a study showing rodents can display increased locomotion up to one year after a single amphetamine dose. Do you think a3b4 antagonists would have utility for former addicts who are currently abstaining but still having issues with hyper-excitability?
There is evidence that ibogaine therapy wears off over time, and some treatment providers recommend taking ibogaine at 6 month intervals. To the extent that there is some factual basis for their belief, it implies that ibogaine can help abstinent addicts.
Is it possible that addicting drugs and behaviors stimulate a3b4, and thus a3b4 blockade is not just a means to an end that is unrelated to the original cause?
That may be the case with nicotine, but it seems unlikely for most of the other dependence syndromes that ibogaine can supposedly treat. The mechanism whereby opioids, alcohol, cocaine, and amphetamines produce reinforcement is well known. All of those drugs have been shown to act in the VTA or on dopaminergic projections from the VTA. Thinking about possible upstream effects opens up a whole can of worms. For example, how are all of those different pharmacological mechanisms converging on nicotinic signaling? It is also unclear how such a mechanism could come into play with amphetamine, since its affects on DA release are not dependent on DA cell firing (meaning any upstream effect is basically negated by amphetamine's MOA).
I managed to find this study http://www.ncbi.nlm.nih.gov/m/pubmed/24750073/ regarding genetic models but it seems hard to interpret because you would think that even modifying the basal level of a3b4 input to the VTA (or knocking it out) would change the dynamics of the VTA (and therefore withdrawal) regardless of whether the drugs were stimulating a3b4 (I hope that makes sense). In other words, are drugs/behaviors actually affecting the a3b4, or is the a3b4 this somewhat "static" input that doesn't get affected too much by the surrounding brain activity?
What I would take away from that study is that alpha3beta4 seems to contribute to the signs of opioid withdrawal.
You can't necessarily assume there is increased excitability. Another possibility is that alpha3beta4 plays a role in the function of the circuits responsible for expression of the somatic signs of withdrawal.Do we have any idea what's providing input to or increasing the excitability of these a3b4 receptors?
Sort of, yes.Regarding the variable mechanics of nicotinic blockade, is this similar to a slow dissossociating NMDA antagonist vs. something like memantine?
serotonin2A
Bluelighter
- Joined
- Sep 13, 2014
- Messages
- 1,354
The effects you are talking about are more likely to have something to do with relapse in abstinent users. Indeed, if you read the litrerature about stress-induced reinstatement of cocaine use, it is driven by Glu transmission to the VTA. (CRF also plays a role).
The other important thing that the projection probably does is generate craving in response to environmental cues. DA is involed in reward expectation/prediction, and so in addicts there is dopamine release in response to cues associated with drug use. That effect is worsened by the fact that the response to dopamine is sensitized in addicts. So you have a situation where craving and pseudowithdrawal set in everytime an addict is exposed to certain environmental cues. If ibogaine can block that process than that in itself would be the basis for an effective treatment. Of course, ibogaine does appear to do more than just that...
However, for their acute reinforcing effects, all of the drugs have direct/indirect effect on dopaminergic neurons in the VTA. Cocaine and amphetamine cause the neurons to dump dopamine into the synapse in a way that is not dependent on firing rate -- so they completely bypass upstream effects on the habenula. Opioids inhibit GABAergic interneurons in the VTA, which can potentially ramp up DA cell firing into a frenzy. Here to, you have a pretty direct mechanism for affecting dopamine. It is hard to believe that activating a natural reward pathway (which is what you are proposing) is going to make much of a difference in those situations.
Furthermore, there is no real reason to think that all abused substances produce reward via a3b4. The primary mechanism of action of ibogaine isn't blocking drug-induced reward. People have usually detoxed when they take ibogaine. The goal of ibogaine use is to stop people from relapsing, rather than somehow extinguishing the rewarding effects of drugs.
EDIT: The other things to look at are ICSS and intra-cranial self-administration studies. If intra-VTA administration of opioids is rewarding and animals wil administer morphine directly into the VTA then that suggests upstream effects do not play a huge role.
The other important thing that the projection probably does is generate craving in response to environmental cues. DA is involed in reward expectation/prediction, and so in addicts there is dopamine release in response to cues associated with drug use. That effect is worsened by the fact that the response to dopamine is sensitized in addicts. So you have a situation where craving and pseudowithdrawal set in everytime an addict is exposed to certain environmental cues. If ibogaine can block that process than that in itself would be the basis for an effective treatment. Of course, ibogaine does appear to do more than just that...
However, for their acute reinforcing effects, all of the drugs have direct/indirect effect on dopaminergic neurons in the VTA. Cocaine and amphetamine cause the neurons to dump dopamine into the synapse in a way that is not dependent on firing rate -- so they completely bypass upstream effects on the habenula. Opioids inhibit GABAergic interneurons in the VTA, which can potentially ramp up DA cell firing into a frenzy. Here to, you have a pretty direct mechanism for affecting dopamine. It is hard to believe that activating a natural reward pathway (which is what you are proposing) is going to make much of a difference in those situations.
Furthermore, there is no real reason to think that all abused substances produce reward via a3b4. The primary mechanism of action of ibogaine isn't blocking drug-induced reward. People have usually detoxed when they take ibogaine. The goal of ibogaine use is to stop people from relapsing, rather than somehow extinguishing the rewarding effects of drugs.
EDIT: The other things to look at are ICSS and intra-cranial self-administration studies. If intra-VTA administration of opioids is rewarding and animals wil administer morphine directly into the VTA then that suggests upstream effects do not play a huge role.
Last edited:
adder
Bluelighter
- Joined
- Mar 28, 2006
- Messages
- 2,851
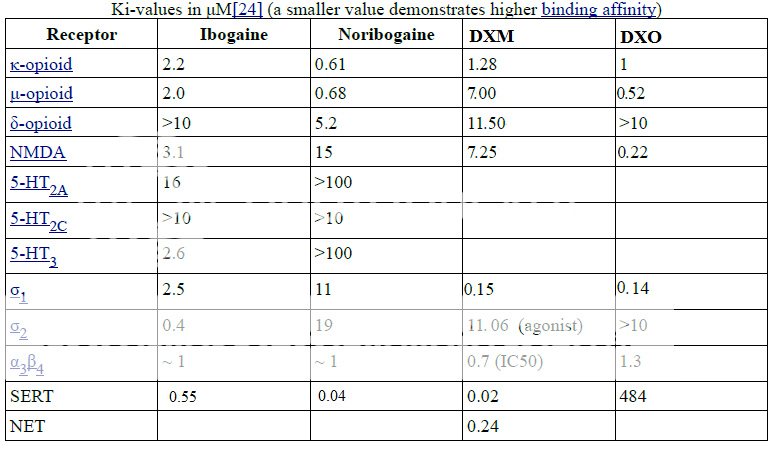
The top values for DXM look like they're in nM (?). Also, isn't nor-DXM meant to be DXO? The value for NMDA receptors looks like that of dextrorphan's (which is ~200-400 nM depending on the source). I've never really seen any affinities for nor-DXM. The affinities of ibogaine and noribogaine at SERT are 0.55 and 0.04 uM respectively (source).
serotonin2A
Bluelighter
- Joined
- Sep 13, 2014
- Messages
- 1,354
The values for ibogaine are in uM. For DXM, it looks like some values are in nM while others are in uM (I doubt the SERT affinity is 20 pM). You are almost certainly correct about DXO. Some people think desmethyl = nor but that is obviously wrong.The top values for DXM look like they're in nM (?). Also, isn't nor-DXM meant to be DXO? The value for NMDA receptors looks like that of dextrorphan's (which is ~200-400 nM depending on the source). I've never really seen any affinities for nor-DXM. The affinities of ibogaine and noribogaine at SERT are 0.55 and 0.04 uM respectively (source).
Last edited:
Cotcha Yankinov
Bluelight Crew
- Joined
- Jul 21, 2015
- Messages
- 2,952
The primary mechanism of action of ibogaine isn't blocking drug-induced reward
I absolutely agree with this now and see your points. I now think most drugs/behaviors acute effects probably have extremely little to do with a3b4, but I'm curious what you think about long term drug use/behaviors (everything from music addiction to sex addiction) stimulating the habenula/VTA glutamate projection little by little, slowly increasing the strength of that glutamate to the point where it is pathological in some fashion. So not that drugs are stimulating a3b4 and that's mediating reward, but rather they are stimulating it a tiny bit (Mostly thinking of MDMA with it's oxytocin effects occurring at least partially in the MPOA and then also sexual effects which seem to occur in the MPOA) and this contributes to strengthing of the cravings and possibly other effects on neurological function? (seems the habenula is extremely involved with sleep).
I must state I am confused at this point regarding how environmental cues would work for things other than visual cues (addiction to audio is what I'm really wondering about) and also how a craving may manifest itself when it's not regarding ingesting a substance in a physical sense, but rather something like internally generated audio. But for example, lets say that someone's cue is a physical sensation - a drinking straw, because when they put their mouth on it, it is similar to a pipe. Now consciously they are feeling their lips against something all the time. So lets say that someone was so sensitized that even just the basal input of feeling their lips 24/7 without anything touching them was an environmental cue. If there is a basal, low level of sensory input 24/7, I assume there would be a craving to match that sensory input of the lips. Now the craving is altering the conscious experience so that the person desires to use a pipe to get high, but what happens when a person is craving audio, and the person is used to "scratching this itch" by playing audio in their head?
Is the general idea something like - a basal, low level signal (cue) constantly runs from the audio cortex to the habenula to the VTA to generate craving, then that VTA signal must then pass to the audio cortex in order for the once low level cue to have any effect on whether audio is produced in an attempt to reduce cravings, then when audio is produced internally it creates fulfillment by linking back into some reward center via direct or indirect (through the cortex?) paths?
I pretty much compulsively play music in my head, and I would hope an a3b4 antagonist would offer some relief... Naltrexone has offered some small relief, but I wish I could say definitively that that was because of MOR blockade.
I think you're right to use amphetamines that blatantly supercede a3b4 as an example and I'll use them to illustrate my refined ponder, that maybe a3b4 is mediating craving as you say, but in a "high resolution" sense, and what I mean by that is depending on what exactly gets activated in the MPOA from other brain regions you're going to be developing/triggering craving via a slightly different pathway through that habenula/VTA glutamate projection - otherwise how could the habenula/VTA glutamate projection correctly differentiate cravings (wanting heroin vs. wanting to eat as a random example, excuse me if hunger is not mediated that way. Also as an aside, if the projection wasn't "high resolution" do you think we'd find people who are super sensitized to craving food after addicting drug abuse? Does this sort of thing happen?).
Essentially amphetamine's increase in dopamine concentrations in the MPOA is probably playing a very small role in amphetamine's overall effects, but if you selectively block dopamine in the MPOA, you get reduced sexual motivation and such (http://www.ncbi.nlm.nih.gov/pubmed/2054609/) Now if an increase in the MPOA's dopamine from amphetamine might increase sexual motivation, and that links back into stimulation of the habenula/VTA glutamate projection, then anything that triggers that specific part of the MPOA might trigger a craving for sex?
Last edited:
serotonin2A
Bluelighter
- Joined
- Sep 13, 2014
- Messages
- 1,354
To give a brief answer, the habenula receives input from PFC, meaning it can receive input with multisensory integration.
In terms of how the brain disentangles the stimulus causing craving, someone who is addicted to cocaine (as an example) and experiences craving in a particular environment will also have formed strong sensory associations between that environment and cocaine. So they will have memories or doing cocaine in the environment, and when they return to the same place, the features in the environment are the thing that triggers VTA DAergic activity in expectation of reward. They recognize the environment as being linked to cocaine and that is what causes the resulting feeling to be interpreted as cocaine craving. The integration of all of these things is done in the same way as sensory binding -- ie, the brain has to be able to process all of the features of a complex stimulus individually but somehow link everything back together.
In terms of how the brain disentangles the stimulus causing craving, someone who is addicted to cocaine (as an example) and experiences craving in a particular environment will also have formed strong sensory associations between that environment and cocaine. So they will have memories or doing cocaine in the environment, and when they return to the same place, the features in the environment are the thing that triggers VTA DAergic activity in expectation of reward. They recognize the environment as being linked to cocaine and that is what causes the resulting feeling to be interpreted as cocaine craving. The integration of all of these things is done in the same way as sensory binding -- ie, the brain has to be able to process all of the features of a complex stimulus individually but somehow link everything back together.
Last edited:
Cotcha Yankinov
Bluelight Crew
- Joined
- Jul 21, 2015
- Messages
- 2,952
To give a brief answer, the habenula receives input from PFC, meaning it can receive input with multisensory integration.
In terms of how the brain disentangles the stimulus causing craving, someone who is addicted to cocaine (as an example) and experiences craving in a particular environment will also have formed strong sensory associations between that environment and cocaine. The integration of all of these things is done in the same way as sensory binding -- ie, the brain has to be able to process all of the features of a complex stimulus individually but somehow link everything back together.
Interesting... I wonder what to make of the increased excitability observed in MDMA user's lateral geniculate nucleus then, because it sounds like the medial geniculate nucleus (" audio hypothalamus") is pretty much the relay for audio, and it's right next to the lateral geniculate nucleus??? Hmmmmmmm
http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3079831/ "We used 3 T functional magnetic resonance imaging during visual stimulation to compare visual system lateral geniculate nucleus (LGN) and Brodmann Area (BA) 17 and BA 18 activation in 20 long abstinent (479.95±580.65 days) MDMA users and 20 non-MDMA user controls. Lifetime quantity of MDMA use was strongly positively correlated with blood oxygenation level-dependent (BOLD) signal intensity in bilateral LGN (rs=0.59; p=0.007), BA 17 (rs=0.50; p=0.027), and BA 18 (rs=0.48; p=0.031), and with the spatial extent of activation in BA 17 (rs=0.059; p=0.007) and BA 18 (rs=0.55; p=0.013). There were no between-group differences in brain activation in any region, but the heaviest MDMA users showed a significantly greater spatial extent of activation than controls in BA 17 (p=0.031) and BA 18 (p=0.049)."
Any comments on the significance of the changes observed?
It sounds like (from what the author was saying) the low level MDMA users actually had decreased excitability which depending on how you interpret the data could obscure the heavy MDMA user's apparently more dramatic increase in excitability.
Last edited:
serotonin2A
Bluelighter
- Joined
- Sep 13, 2014
- Messages
- 1,354
Interesting... I wonder what to make of the increased excitability observed in MDMA user's lateral geniculate nucleus then, because it sounds like the medial geniculate nucleus (" audio hypothalamus") is pretty much the relay for audio, and it's right next to the lateral geniculate nucleus??? Hmmmmmmm.
The thalamus is the relay that gates some types of input to cortex. That includes sensory input -- the thalamic nuclei allow the cortex to direct attention to particular stimuli and filter out extraneous info. But I'm not following the relevance of this to the earlier discussion RE ibogaine...
FYI, I went back to my last post and made an edit to clarify my response. Hopefully the process will make more sense.
Last edited:
You're absolutely right: DXM and DXO's NMDA/OP/sigma Ki values are in nM (instead of uM). So should read:The top values for DXM look like they're in nM (?). Also, isn't nor-DXM meant to be DXO? The value for NMDA receptors looks like that of dextrorphan's (which is ~200-400 nM depending on the source). I've never really seen any affinities for nor-DXM. The affinities of ibogaine and noribogaine at SERT are 0.55 and 0.04 uM respectively (source).
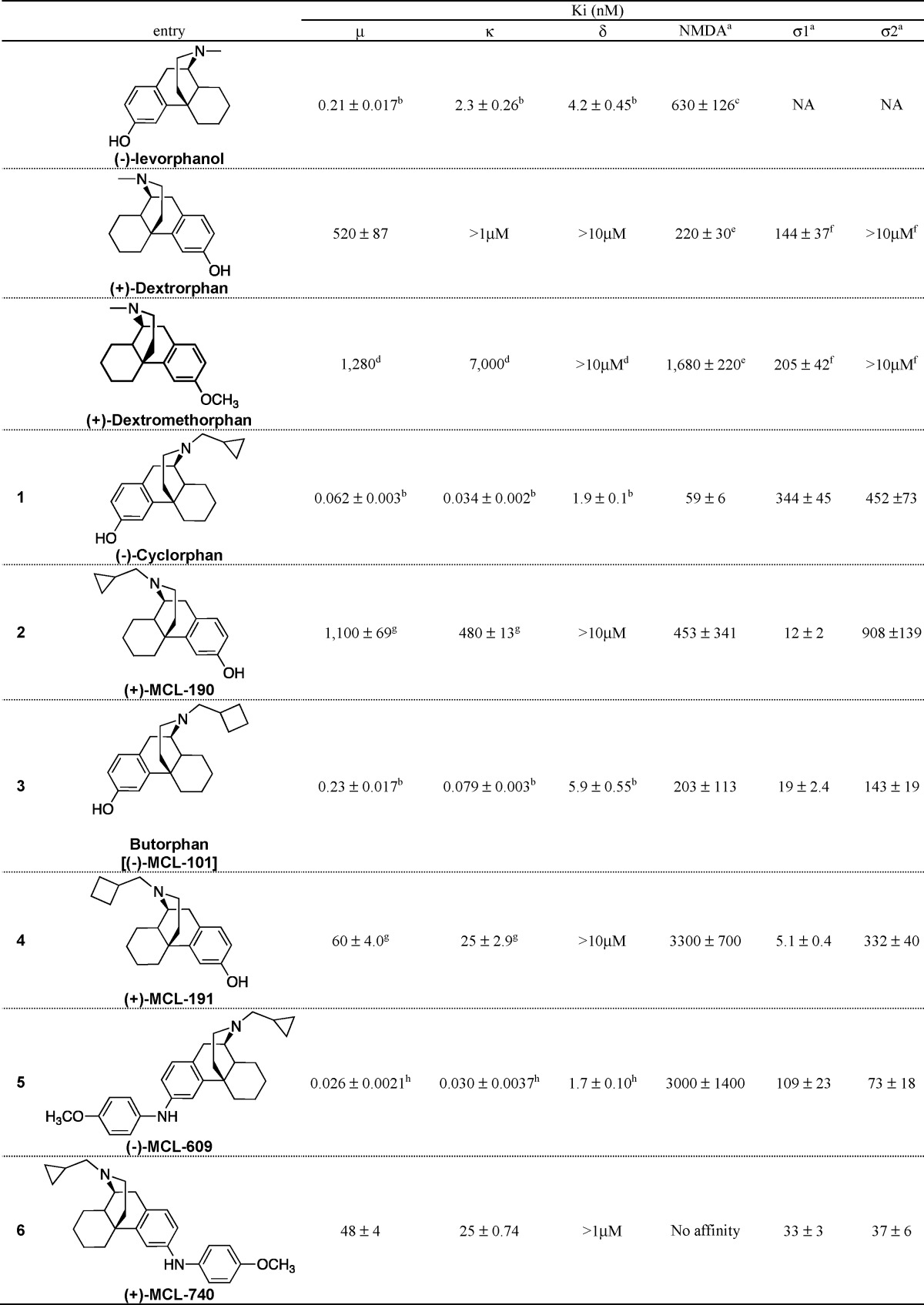
Here is the source: "Preliminary Pharmacological Evaluation of Enantiomeric Morphinans ACS Chem Neurosci. 2014 Feb 19; 5(2): 93–99.
Nor-DMX = DXO ie (+)Dextrorphan. ..
Now that you point that out, I am even more convinced Ibogaine and DXM are pretty much same pharmacologically.. will come to back to that topic later...





