-
Neuroscience & Pharmacology Discussion Welcome Guest
Posting Rules Bluelight Rules Recent Journal Articles Chemistry Mega-Thread FREE Chemistry Databases! Self-Education Guide -
N&PD Moderators: Skorpio | someguyontheinternet
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
I Like to Draw Pictures of Random Molecules
- Thread starter nuke
- Start date
- Status
- Not open for further replies.
Question about planar rings and such; couldn't cyclohexadiene work as an isostere? It's a lot flatter than cyclohexane:
Amphetamine: https://i.imgur.com/wiFudTm.png
1,4-Cyclohexadiene analog: https://i.imgur.com/ipiNJDU.png
Cyclohexane analog: https://i.imgur.com/4TlqmpW.png
You have the hydrogens on the saturated carbons sticking out of plane but the rest is flat. It's not aromatic of course but I'm curious as to how it would work out.
Amphetamine: https://i.imgur.com/wiFudTm.png
1,4-Cyclohexadiene analog: https://i.imgur.com/ipiNJDU.png
Cyclohexane analog: https://i.imgur.com/4TlqmpW.png
You have the hydrogens on the saturated carbons sticking out of plane but the rest is flat. It's not aromatic of course but I'm curious as to how it would work out.
Last edited:
Nagelfar
Bluelight Crew
Question about planar rings and such; couldn't cyclohexadiene work as an isostere? It's a lot flatter than cyclohexane:
Amphetamine: https://i.imgur.com/wiFudTm.png
1,4-Cyclohexadiene analog: https://i.imgur.com/ipiNJDU.png
Cyclohexane analog: https://i.imgur.com/4TlqmpW.png
You have the hydrogens on the saturated carbons sticking out of plane but the rest is flat.
EDIT: Nevermind, I was thinking benzene, yes cyclohexadiene is closer to benzene than cyclohexane. Some of those cyclopentane isostere have a few hydrogens out of plane too, so I'd venture it a possibility depending on where and how it needs to fit.
belligerent drunk
Bluelight Crew
- Joined
- Sep 16, 2015
- Messages
- 3,482
Yeah it's an intermediate.. but I can check Shulgin's last big book to see if I see any PEA's with an extra methylene spacer.. (let alone an aldoxime, which just seems reactive))?
It would most likely hydrolyze into the respective aldehyde and hydroxylamine. The aldehyde would probably not be very useful.
Question about planar rings and such; couldn't cyclohexadiene work as an isostere? It's a lot flatter than cyclohexane:
Amphetamine: https://i.imgur.com/wiFudTm.png
1,4-Cyclohexadiene analog: https://i.imgur.com/ipiNJDU.png
Cyclohexane analog: https://i.imgur.com/4TlqmpW.png
You have the hydrogens on the saturated carbons sticking out of plane but the rest is flat. It's not aromatic of course but I'm curious as to how it would work out.
cyclohexadiene is unstable under normal conditions: easily oxidized by air to give the corresponding benzene (driving force = aromaticity energy gain). This is the reason why products of such compounds always contain [Oxidation] inhibitor such as BHT. But if one of the two sp3 carbons of the cyclohexadiene is di-substituted (say by 2 methyls), then it should be fine. But disubstitution of one of these carbons will make you loose even more in terms of planarity of the system).
adder
Bluelighter
- Joined
- Mar 28, 2006
- Messages
- 2,851
If you want to replace the cyclohexyl in ketamine with a phenyl ring, then it can't have the keto moiety.
You can't have both the amine and the aryl group either, you end up with biphenyl or a substituted aniline.
Jaw Clenching
Bluelighter
- Joined
- Feb 4, 2005
- Messages
- 551
Thanks for the input @solipsis
It seems that I am on a kick with -FLY derivatives. They interest me a lot. I was wondering if any of the following -FLY derivative would be in theory psychoactive at all.
Thanks!
Did you see these ones I came up with?
http://www.bluelight.org/vb/threads...-molecules?p=13637715&viewfull=1#post13637715
http://www.bluelight.org/vb/threads...-molecules?p=13637708&viewfull=1#post13637708
http://www.bluelight.org/vb/threads...-molecules?p=13637699&viewfull=1#post13637699
Not sure if some of those are even possible to make.
I couldn't find any molecules that have a Pyrrole group attached to an Indole group - basically di-indole or something.
Here's an example to better visualize what I'm saying:
Nagelfar
Bluelight Crew
Question about planar rings and such; couldn't cyclohexadiene work as an isostere? It's a lot flatter than cyclohexane:
Amphetamine:
1,4-Cyclohexadiene analog:
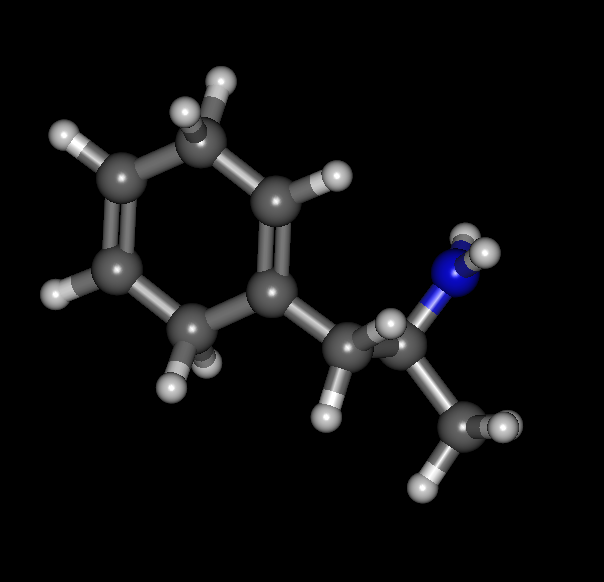
Cyclohexane analog:
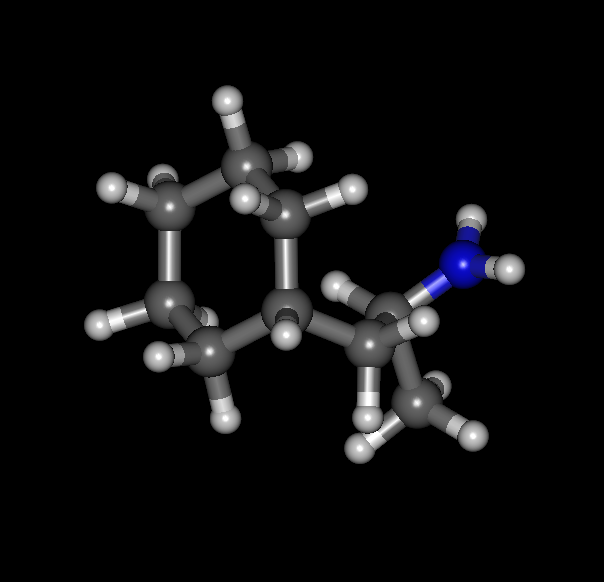
You have the hydrogens on the saturated carbons sticking out of plane but the rest is flat. It's not aromatic of course but I'm curious as to how it would work out.
cyclohexadiene is unstable under normal conditions: easily oxidized by air to give the corresponding benzene (driving force = aromaticity energy gain). This is the reason why products of such compounds always contain [Oxidation] inhibitor such as BHT. But if one of the two sp3 carbons of the cyclohexadiene is di-substituted (say by 2 methyls), then it should be fine. But disubstitution of one of these carbons will make you loose even more in terms of planarity of the system).
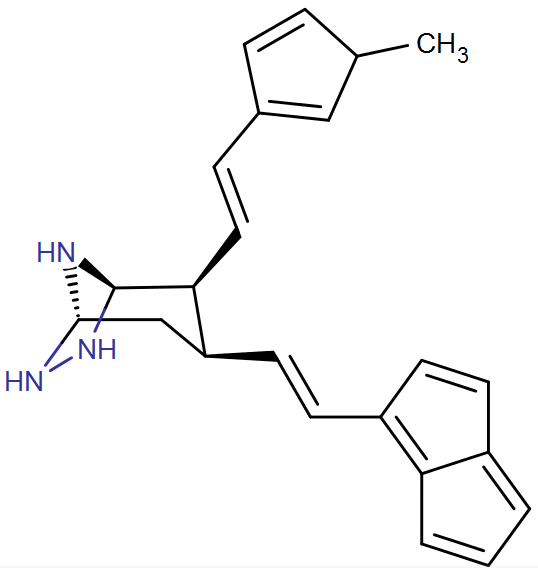
Check out the two position, though the cyclohexadiene idea is on the three position where the arene is.
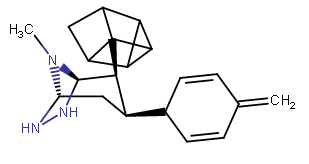
it looked like this:
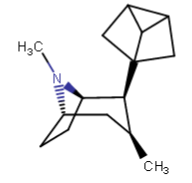
...and, ironically, made the H saturated angles connected with the hope that it would come out more planar with a single carbon linking two more internal/inward-facing methylenes:
(not thinking, that duh, the one carbon between two linkers is CH2 still; could just double-bond those two but the 2D result isn't as aesthetically pleasing for a random molecule to show off)
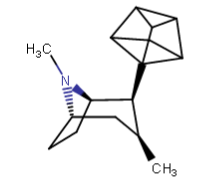
and upon "cleaning" it got the rectangular box of the first image. Is that thing even physically possible? Tenable?
And finally:
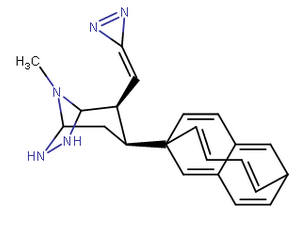
Some kinda naphthdiene-endo-ethendio
...if you 'clean up' the image automatically in Marvin Beans.... it transforms into
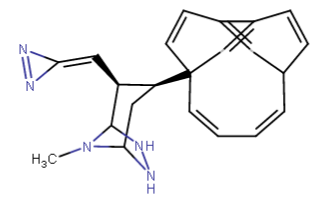
...Gengar the Pokémon:

belligerent drunk
Bluelight Crew
- Joined
- Sep 16, 2015
- Messages
- 3,482
You can't have both the amine and the aryl group either, you end up with biphenyl or a substituted aniline.
Yeah I didn't consider that the carbon is already quaternary. My bad.
Bagseed
Bluelighter
at least this one won't be possible in my opinion. sp2 hybrid orbitals are oriented in a planar fashion. so you cannot have these bonds from the C=C sticking downwards the way they do. energetically extremely unfavorable.
also this diazo group forming the 3-ring will be extremely reactive and might disintegrate very fast to release nitrogen gas (possibly leaving behind a carbene which is very reactive and might turn the olefine into an alkine).
Nagelfar
Bluelight Crew
at least this one won't be possible in my opinion. sp2 hybrid orbitals are oriented in a planar fashion. so you cannot have these bonds from the C=C sticking downwards the way they do. energetically extremely unfavorable.
also this diazo group forming the 3-ring will be extremely reactive and might disintegrate very fast to release nitrogen gas (possibly leaving behind a carbene which is very reactive and might turn the olefine into an alkine).
Yeah well it was rather fatuitous, as my work if oft meant to be; but what of the one above it?
Bagseed
Bluelighter
well, to be sure one would have to model the exact bond angles of that thing... sp3 carbon is most comfortable with a nice tetrahedral (~109° bond angles) structure, I guess there will be some strain on the bonds, but I have no idea if it will be practically possible.
Nagelfar
Bluelight Crew
Back to malmet's cyclohexadiene idea; would this be stable? -
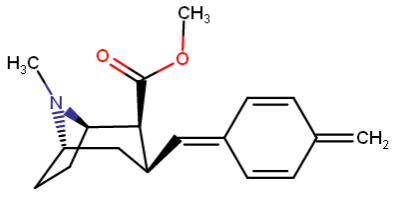

Nagelfar
Bluelight Crew
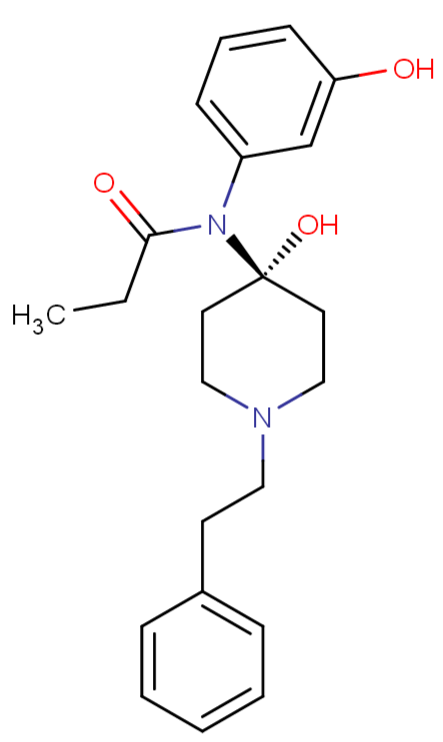
Fentanyl + O-desmethyl-tramadol (the methoxys)
- Status
- Not open for further replies.