sekio
Bluelight Crew
Why so?
no/very little steric hindrance around the two outer carbons (if you count from the top as #1 they are the 3rd and 4th members of the ring)
look up the mechanism of acetal hydrolysis
also enzymes in biological systems can catalyse this rxn just as well as H+, presumably also certain metals/ions, etc
you are right that the ethylene glycol generated wouldnt be toxic but the hydrolysis of the ring would destroy the activity
a better analog for the ester group (die Ester-Gruppen??) would be an oxazoline or whatever is present in that one RTI compound - a ring just like that acetal except with a nitrogen with a double bond in it present in the place of one of the oxygens






























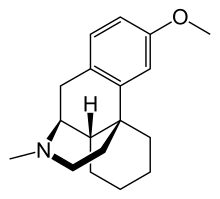








 ) "pure" DXM-like dissociative with no opioid to worry about! may be some stim?? but pretty safe I would imagine!
) "pure" DXM-like dissociative with no opioid to worry about! may be some stim?? but pretty safe I would imagine!