sekio
Bluelight Crew
but what about analogs of morpheus, 5-meo-morpheus, 4-ho-morpheus, etc
N&PD Moderators: Skorpio | someguyontheinternet
What do you think about these molecules?




There's a paper on 2C-B-Norphenmetrazine somewhere out there. Kinda annoying synthesis, like 5 steps from bk-2C-B iirc...


is bad news (like hospital news) at or above 100mg, which admittedly doesn't say anything much about yours necessarily.
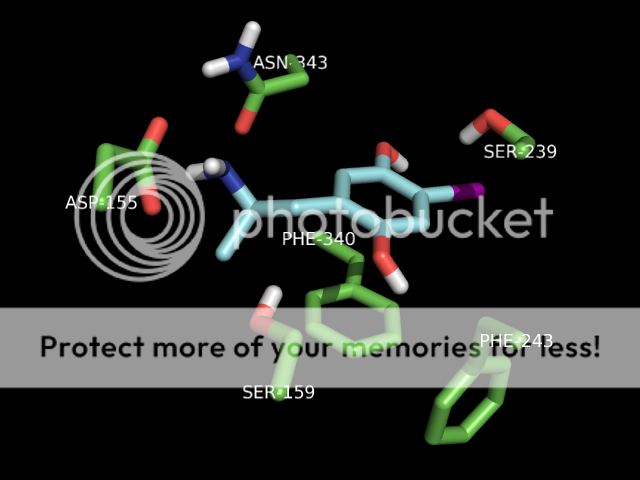
Actually simple imidazole pKa = 6.99 at 25 °C (CRC Handbook of Chemistry & Physics) so it is way better leaving group than RO-. Not as good as Halo or even acetate (pka 3-5) but under the right conditions (eg with relatively strong nucleophiles) carbonyl imidazoles will react. Thats' why for example carbonyl-diimidazole (Im2C=O) is used as safer replacement of phosphege (Cl2C=O) to acylate amines or activated carboxylic acids. But yeah you're right the presence of the other nitrogen will certainly make this molecule even less reactive. But my take is that primary amines in protein (eg lysines - relatively strong nucleophile) may react and give irreversibly acylated protein. Under the right conditions! But who knows? hard to explain why this guy has his brain fried by this molecule!
You are absolutely right on the pKa values because imidazoles are amphoteric (base and acid) so naturally you'll have 2 pKas. But the pKa that matter for the reaction I was mentionning is that of the the acid: ImH+ <------> ImH . The reason being at physiological pH (7.4) major form is ImH+ (not that much but at least 20%more than the neutral form). Like this in the case of acyl-imidazoles like in the cannabinoid molecule :The pKa you describe is the one for imidazole which is already protonated, that is to say: imidazole(H+) <---> imidazole + H+; pKa=6.99
However, imidazole <-----> imidazole- + H+; pKa = 14.5. The only reason why the pKa of this acid-base reaction is way less than it should be (see ammonia pKa = 32.5) is because when deprotonated, the lone pair can delocalise into the imidazole ring and Huckel's rule is satisfied (the system gains extra stability from aromaticity).

True Lysines are poor nucleophiles at physiological pH(7.4) but not always. Some lysines in proteins are actually quite good nucleophile by happening to be either in a basic microenvironment where pH is relatively basic (eg in some cell organelles eg lysosomes pH can be as high as 10!!!). Or made "basic" for example by nearby groups eg the Imidiazole =N of histidines. it all depends on the protein structure microenvironment in which the lysine located. BUT you are absolutely right at physiological pH (7.4) most lysines will be protonated therefore not nucleophilic.Also lysines aren't really good nuclephiles because they're pretty much always charged, so the nitrogen lone pair is mostly unavailable. In hydrolytic enzymes, lysine is almost always never used as the nucleophile in the active site. It is normally serine or cysteine (which is first deprotonated by a base like histidine so nucleophilicity of the serine or cysteine residue is increased).