-
Neuroscience & Pharmacology Discussion Welcome Guest
Posting Rules Bluelight Rules Recent Journal Articles Chemistry Mega-Thread FREE Chemistry Databases! Self-Education Guide -
N&PD Moderators: Skorpio | someguyontheinternet
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
I Like to Draw Pictures of Random Molecules
- Thread starter nuke
- Start date
- Status
- Not open for further replies.
Dresden
Bluelighter
- Joined
- Feb 2, 2010
- Messages
- 3,212
I found an instance of a carbomethoxy acting in place of benzene:

Arecoline, a mild stimulant. It's good too; I used it once.
* * *

isoxazole
One lone pair of the oxygen and two electrons a piece from the two double bonds participate in the aromaticity of the ring. The nitrogenous lone pair does not participate in isoxazole's aromaticity. In fact, the N's lone pair of electrons is orthogonal to the aromatic pi ring system electrons.
2 + 2 + 2 = 6 pi electrons
Huckel's Rule
H(n) = 4n + 2
H(1) = 4(1) + 2 = 6, making isoxazole a 1st order (n=1) aromatic compound.
Sorry, that was actually an easy question once I looked at it.

Arecoline, a mild stimulant. It's good too; I used it once.
* * *

isoxazole
One lone pair of the oxygen and two electrons a piece from the two double bonds participate in the aromaticity of the ring. The nitrogenous lone pair does not participate in isoxazole's aromaticity. In fact, the N's lone pair of electrons is orthogonal to the aromatic pi ring system electrons.
2 + 2 + 2 = 6 pi electrons
Huckel's Rule
H(n) = 4n + 2
H(1) = 4(1) + 2 = 6, making isoxazole a 1st order (n=1) aromatic compound.
Sorry, that was actually an easy question once I looked at it.
Last edited:
aced126
Bluelighter
- Joined
- May 18, 2015
- Messages
- 1,047
I found an instance of a carbomethoxy acting in place of benzene:

Arecoline, a mild stimulant. It's good too; I used it once.
* * *

isoxazole
One lone pair of the oxygen and two electrons a piece from the two double bonds participate in the aromaticity of the ring. The nitrogenous lone pair does not participate in isoxazole's aromaticity. In fact, the N's lone pair of electrons is orthogonal to the aromatic pi ring system electrons.
2 + 2 + 2 = 6 pi electrons
Huckel's Rule
H(n) = 4n + 2
H(1) = 4(1) + 2 = 6, making isoxazole a 1st order (n=1) aromatic compound.
Sorry, that was actually an easy question once I looked at it.
Thanks, is there any way of determining in a case where more than one heteroatom has lone pairs to donate into the ring, which lone pair actually delocalises?
Is arecoline amphetamine-like in action? The wikipedia page says it's a partial muscinaric agonist.
belligerent drunk
Bluelight Crew
- Joined
- Sep 16, 2015
- Messages
- 3,482
Thanks, is there any way of determining in a case where more than one heteroatom has lone pairs to donate into the ring, which lone pair actually delocalises?
Is arecoline amphetamine-like in action? The wikipedia page says it's a partial muscinaric agonist.
What rings do you have in mind? Anyway, if a heteroatom had a lone pair and it was not delocalized into the ring structure (and it wasn't double-bonded to a neighbor), then that would break aromaticity as there can be no atoms in the chain that don't have a pi orbital aligned with the ring's delocalized pi electron cloud. Same principle as with conjugated double bonds - if there is a sp3 carbon somewhere in the middle then it breaks conjugation. If a heteroatom is double-bonded to its neighbor, then its lone pair is free and not conjugated; if it doesn't have a double bond within the ring, then it must donate its lone pair.
aced126
Bluelighter
- Joined
- May 18, 2015
- Messages
- 1,047
What rings do you have in mind? Anyway, if a heteroatom had a lone pair and it was not delocalized into the ring structure (and it wasn't double-bonded to a neighbor), then that would break aromaticity as there can be no atoms in the chain that don't have a pi orbital aligned with the ring's delocalized pi electron cloud. Same principle as with conjugated double bonds - if there is a sp3 carbon somewhere in the middle then it breaks conjugation. If a heteroatom is double-bonded to its neighbor, then its lone pair is free and not conjugated; if it doesn't have a double bond within the ring, then it must donate its lone pair.
For example, why isn't 1,4-diazepine aromatic? It has 6 pi electrons from the double bonds itself, and 2 lone pairs on the nitrogens. Why isn't pyran aromatic? Why isn't oxepine or thiepine aromatic? Can't both the oxygen lone pairs delocalise into the ring to make 10 pi electrons?
belligerent drunk
Bluelight Crew
- Joined
- Sep 16, 2015
- Messages
- 3,482
According to Pauli principle only 2 electrons can occupy the same space, so only 1 pair can be aligned with the ring electron structure; 4 electrons can't be in the same space thus the other pair/orbital has to be somewhere else, which is why p orbitals face different directions.
1,4-diazepine isn't aromatic because it has 8 pi electrons in the ring which makes it antiaromatic (4n is antiaromatic, 4n+2 is aromatic; a general rule), same goes for oxepine and thiepine. Pyran isn't aromatic because there's one sp3 carbon in the ring which means that the conjugation is "broken", like it's missing a link. Furan on the other hand is aromatic with all carbons being sp2 giving 4 pi electrons plus oxygen's pair, totaling 6 pi electrons.
1,4-diazepine isn't aromatic because it has 8 pi electrons in the ring which makes it antiaromatic (4n is antiaromatic, 4n+2 is aromatic; a general rule), same goes for oxepine and thiepine. Pyran isn't aromatic because there's one sp3 carbon in the ring which means that the conjugation is "broken", like it's missing a link. Furan on the other hand is aromatic with all carbons being sp2 giving 4 pi electrons plus oxygen's pair, totaling 6 pi electrons.
adder
Bluelighter
- Joined
- Mar 28, 2006
- Messages
- 2,851
If the carbomethoxy binds to the same site as the phenyl, then wouldn't this
do something good?
Perhaps it would have some weak stimulant activity like this DMAA designer drug. But it's not like the carbomethoxy in phenyltropanes or cocaine interacts with the Y156 at the same angle and distance as the phenyl in amphetamine does. The fact that it helps with affinity in phenyltropanes doesn't mean that phenyltropanes could do without the phenyl ring.
If this is so then what does the phenyl in phenyltropanes do? So the reason why amphetamine dissociates from DAT once inside the neuron is because the interactions between the amph pi cloud and the tyrosine pi cloud are weak? But the carbomethoxy (or bioisostere) has strong enough binding to the tyrosine pi cloud/hydroxyl itself to prevent dissociation once exposed to the presynaptic membrane? Or maybe the carbomethoxy binding instead of the phenyl means the phenyl can interfere in the conformational change the transporter undergoes when in the process of transportation of substrate from synaptic cleft to cytosol?
The phenyl ring in phenyltropanes likely interacts with the same tyrosine residue that the carbomethoxy does. And the positioning of the phenyl ring in phenyltropanes when they're bound is certainly different from that in amphetamine, you can see that the same substituent on the phenyl ring can have different effect in phenyltropanes and amphetamines. As I imagine it, you can never judge the ability of a compound to bind to its target by considering single interactions between moieties in the compound's molecule and aminoacid residues, binding clefts in receptors can be quite tight, so all steric and electronic effects have to be taken into account. I mean even if it seems like the distance between some residue with an aromatic bit and some positively charged site in the receptor matches the distance between the aromatic ring and the amine in your compound, it doesn't automatically mean that it will bind there, what is present in the neighbourhood is important too. Compounds lacking the ester are less potent stimulants, as clubcard wrote in this thread or the other one, that 4-nitro compound is a few times weaker than cocaine, right?
I don't have article names at hand, but you can search for dopamine, CFT & amphetamine binding at DAT. There are many articles explaining how these molecules bind to DAT and they have nice graphics showing it.
Dresden
Bluelighter
- Joined
- Feb 2, 2010
- Messages
- 3,212
Afrin (oxymetazoline) inspired speed:

Afrin (oxymetazoline) inspired ecstasy:

I guess it's kind of obvious from my posts that two of my favorite drugs are methamphetamine and MDMA.
* * *
Yeah, chewing on betel nut (whose active ingredient is arecoline) gives a mildly stimulating, strangely rewarding yet subtle high.

Afrin (oxymetazoline) inspired ecstasy:

I guess it's kind of obvious from my posts that two of my favorite drugs are methamphetamine and MDMA.
* * *
Yeah, chewing on betel nut (whose active ingredient is arecoline) gives a mildly stimulating, strangely rewarding yet subtle high.
Last edited:
Fruitofknowledge
Greenlighter
- Joined
- Sep 30, 2015
- Messages
- 28
Yeah there a containment in street meth when people utilize the birch reduction and add too much lithium.
Fruitofknowledge
Greenlighter
- Joined
- Sep 30, 2015
- Messages
- 28
Desdihydromethamphetamines.
Dresden
Bluelighter
- Joined
- Feb 2, 2010
- Messages
- 3,212
Desdihydromethamphetamines.
Umm...do you mean a partially reduced benzene ring?
pr0d1gy
Bluelighter
On a different topic, which of the lone pairs in iso-oxazole is donated into the ring? One of the 2 oxygen lone pairs or the nitrogen lone pair?
In isoxazoles it would be oxygen. Nitrogen is already contributing one electron to the pi system so it can't contribute the lone pair as well, among other reasons.
Last edited:
Midnight Sun
Bluelighter

What would this do
slightly less crappy version of ppa/ephedrine?
which isn't saying much either
aced126
Bluelighter
- Joined
- May 18, 2015
- Messages
- 1,047
slightly less crappy version of ppa/ephedrine?
which isn't saying much either
Would me more than "slightly less crappy"; compare said structure to phenmetrazine.
Midnight Sun
Bluelighter
Would me more than "slightly less crappy"; compare said structure to phenmetrazine.
It's far closer to ppa/ephedrine than phenmetrazine, maybe you could expect a jittery methcathinone type high
beta-methoxy = adrenergic BS
That shit will love the NET
Dresden
Bluelighter
- Joined
- Feb 2, 2010
- Messages
- 3,212
Re-uptake inhibitor or neurotransmitter releaser or both (is that even possible)?

This next one is the bom:

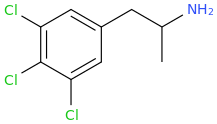
SHIVA

N-Me-SHIVA

N-Et-SHIVA
N-ethyl-SHIVA looks as silky smooth as getting fucked in the ass while lubed up with a silicone based lubricant.

PARVATI
Haha, checkmate.

This next one is the bom:


SHIVA

N-Me-SHIVA

N-Et-SHIVA
N-ethyl-SHIVA looks as silky smooth as getting fucked in the ass while lubed up with a silicone based lubricant.

PARVATI
Haha, checkmate.
Last edited:
- Status
- Not open for further replies.





