-
Neuroscience & Pharmacology Discussion Welcome Guest
Posting Rules Bluelight Rules Recent Journal Articles Chemistry Mega-Thread FREE Chemistry Databases! Self-Education Guide -
N&PD Moderators: Skorpio | someguyontheinternet
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
I Like to Draw Pictures of Random Molecules
- Thread starter nuke
- Start date
- Status
- Not open for further replies.
Nagelfar
Bluelight Crew

Now that I got that Bentley frame for this latest vehicle of my molecular masturbation, let's see how it does along-side any ol' rig. ;p
Nagelfar
Bluelight Crew

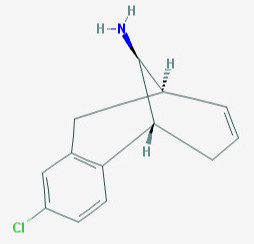
The above are Metazocine (on left) & Org 6582 (to right); the former an opioid and latter a MAT reuptake inhibitor. Any idea from anyone on how to go about crossing these two in a way that keeps both functionalities? So very similar.
EDIT:
Here's my feeble attempt, but all I did was merge all of the features of both; if the chloro or oxy, for instance, hinders the functionality of the other, this is then untenable, but nonetheless, this is my go at it:

Last edited:
neurotic
Bluelighter
scratch those last ones i drew... i looked at a few more pipradrol analogues and non-phenanthrene opioids. here's my second take at pipradrol/phenidate analogues with opioid activity. i'm with Nagelfar looking for opioids with MAT activity here...

the second one is just MPH and pipradrol mixed, probably no opioid activity but might be a stimulant who knows. i tweaked the piperidinyl and added a methyl group on the nitrogen hopefully for opioid activity below.

hopefully they also work as euphoric transporter inverse agonists like cocaine and MPH and not just seizure inducing reuptake inhibitors à la pethidine.
wondering how one could add some serotonin releasing properties as well, it could be called MDMorphine

the second one is just MPH and pipradrol mixed, probably no opioid activity but might be a stimulant who knows. i tweaked the piperidinyl and added a methyl group on the nitrogen hopefully for opioid activity below.

hopefully they also work as euphoric transporter inverse agonists like cocaine and MPH and not just seizure inducing reuptake inhibitors à la pethidine.
wondering how one could add some serotonin releasing properties as well, it could be called MDMorphine

Last edited:
neurotic
Bluelighter
^ i get a pop up saying "this drug is not available in your country!"
had a few more tries at a stimulant and opioid hybrid, starting from the phenyltropane skeleton this time

had a few more tries at a stimulant and opioid hybrid, starting from the phenyltropane skeleton this time

neurotic
Bluelighter
that metazocine and Org hybrid confused the fuck out of me

here's an easier on the eyes view on it and its parents, FWIW

here's an easier on the eyes view on it and its parents, FWIW
Nagelfar
Bluelight Crew
that metazocine and Org hybrid confused the fuck out of me

here's an easier on the eyes view on it and its parents, FWIW
Marvin Beans sketch doesn't render the most cleanly, and by the time you've hit "clean", trying to straighten it out manually will just likely create more bonds upon clicking on accident and I just don't bother.
neurotic
Bluelighter
sure, i just thought you might find use for them or something. didnt mean to be annoying about it
Nagelfar
Bluelight Crew
It looks like cocaine "doin' the robot" ;j
conscious-observed
Bluelighter
- Joined
- May 12, 2015
- Messages
- 89
I wish I knew more chemistry....
Midnight Sun
Bluelighter
beta-oxetane, dude
Though I read somewhere that it'd increase polarity further over the cathinones so idfk, could just be shit tier
^^^I'm liking what neurotic's got going on up there... some things reminiscent of diconal :D -- I was playing around with diconal/palfium structure a while back, it'd be fun to get an analogue of em made up sometime
I don't have any chemdrawing app or anything but I have a fairly simple idea - in PIHKAL, Shulgin mentions the lack of modification that can be made to halogen groups. Why not stick the halogen on the end of a carbon chain? i.e. DOM but with a bromomethyl group instead of just a methyl group.
Are there any obvious synthetic or biological barriers to this?
Are there any obvious synthetic or biological barriers to this?
sekio
Bluelight Crew
Are there any obvious synthetic or biological barriers to this?
primary alkyl bromides and iodides tend to be reactive compounds that undergo SN2 reactions in the body, permanently attaching them to e.g. lysine residues and such.
alkyl chlorides/fluorides are more forgiving. (see: clomethiazole, DOEF).
but e.g. stuff like propyl bromide or methyl iodide is suprisingly toxic...
sekio
Bluelight Crew
You don't want the compound to undergo any nucleophilic substitutions at all, ideally.
I also don't think it's "bulk" neccesarily that promotes SN1 over SN2 reactions, rather it's whether or not the alkyl halide is primary, secondary, or tertiary (& hence determining stability of the resulting carbocation intermediate in SN1/E1 reactions)

I also don't think it's "bulk" neccesarily that promotes SN1 over SN2 reactions, rather it's whether or not the alkyl halide is primary, secondary, or tertiary (& hence determining stability of the resulting carbocation intermediate in SN1/E1 reactions)

"Bulk" does affect it. If the carbon is primary/secondary, but significantly sterically hindered, the attacking group simply can't "attack", and the carbocation is unstable, thus reducing the overall reactivity. But then again you'd have to employ a few tricks to make it bulky AND have the carbon primary/secondary, and that would most likely be too much "bulk".
Dresden
Bluelighter
- Joined
- Feb 2, 2010
- Messages
- 3,212
These two, I fear, would be too jittery in classic adrenergic fashion to be much enjoyable, but who knows, maybe they're great:

and

The first one should be a piece of cake to synth in one step from ma huang extract. The second one, well, it's not the easiest synths in the world, but it's well within China's research chemical producing chemists' reach in case anyone wants to order a custom synthesis. (j/k)
Note also that each of these two molecules have two chiral centers a piece leading to a total of 4 possible stereoisomers, which will negatively impact the drugs' potency potency per milligram produced ratio; however, since they are both diastereoisomers, chiral resolution should be much easier than if they were simple one chiral carbon containing enantiomers such as (R/S)-MDMA or (R,S)-methamphetamine, for example.

and

The first one should be a piece of cake to synth in one step from ma huang extract. The second one, well, it's not the easiest synths in the world, but it's well within China's research chemical producing chemists' reach in case anyone wants to order a custom synthesis. (j/k)
Note also that each of these two molecules have two chiral centers a piece leading to a total of 4 possible stereoisomers, which will negatively impact the drugs' potency potency per milligram produced ratio; however, since they are both diastereoisomers, chiral resolution should be much easier than if they were simple one chiral carbon containing enantiomers such as (R/S)-MDMA or (R,S)-methamphetamine, for example.
Last edited:
- Status
- Not open for further replies.











