-
Neuroscience & Pharmacology Discussion Welcome Guest
Posting Rules Bluelight Rules Recent Journal Articles Chemistry Mega-Thread FREE Chemistry Databases! Self-Education Guide -
N&PD Moderators: Skorpio | someguyontheinternet
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
I Like to Draw Pictures of Random Molecules
- Thread starter nuke
- Start date
- Status
- Not open for further replies.
pharmakos
Bluelighter
those sure are gonna smell, but i think they'll be winners

benzofuran version of 4-ho-met, idea from http://en.wikipedia.org/wiki/Dimemebfe

al-lad + lsz + ald-52 modification of lsd, because why not if i'm already making shit up

o-desmethylethyltramadol

2cd-fly

2c-e-fly

2c-al-fly

difmdma
Last edited:
DiFMDA has been made
I think don't think fluorination is the way forward to 'beat' MDMA. I think MDMA is may just be the pinnacle.
I think don't think fluorination is the way forward to 'beat' MDMA. I think MDMA is may just be the pinnacle.
overstoned
Greenlighter
- Joined
- Jul 27, 2010
- Messages
- 7
Would you take this? Pyridine vs phenyl

2-METHYL-1-(3-PYRIDINYL)-1-PROPANONE
Niacin/nicotinamide tweaked out. I presume this would do nothing?
Can someone explain the difference the pyridine vs phenyl would make? I'm sure it's been thought of many times but I am retarded when it comes to certain aspects of biochemistry.
What about 3-isobutylpyridine??

2-METHYL-1-(3-PYRIDINYL)-1-PROPANONE
Niacin/nicotinamide tweaked out. I presume this would do nothing?
Can someone explain the difference the pyridine vs phenyl would make? I'm sure it's been thought of many times but I am retarded when it comes to certain aspects of biochemistry.
What about 3-isobutylpyridine??
sekio
Bluelight Crew
It looks like a cut down analogue of metyrapone, a compound that messes with liver enzymes. It's hard to accurately guess what activity the compound would have based upon its structure alone.
I probably wouldn't go eating random pyridine compounds. Some of them can be carcinogenic or otherwise toxic.
Pyridine has a nitrogen which can hydrogen bond and coordinate to things, it's also polar because of this.
Isobutylpyridine would be a corrosive, nasty liquid that probably has no use as a drug either.
I probably wouldn't go eating random pyridine compounds. Some of them can be carcinogenic or otherwise toxic.
Can someone explain the difference the pyridine vs phenyl would make?
Pyridine has a nitrogen which can hydrogen bond and coordinate to things, it's also polar because of this.
Isobutylpyridine would be a corrosive, nasty liquid that probably has no use as a drug either.
blueberries
Bluelighter
Last edited:
Nice.. I wonder if it would be active
sekio
Bluelight Crew
I'd expoect the methoxy groups to fall off under acidic conditions, they are hemiaminal ethers. At least when it's 2 oxygens, it decomposes to formaldehyde + 2 alcohols.
What we really need is for science and engineering to advance to a point where you'd draw a molecule in chemdraw, run a simulation (in reasonable time on a single pc), and it would calculate all of it's binding affinities, downstream effects, metabolites, ...
pharmakos
Bluelighter
What we really need is for science and engineering to advance to a point where you'd draw a molecule in chemdraw, run a simulation (in reasonable time on a single pc), and it would calculate all of it's binding affinities, downstream effects, metabolites, ...
i hope that doesn't happen any time soon.... at least not until drug laws are less barbaric.
if that ever happens it would be way too easy for blanket bans to happen.... i.e. "any synthetic molecule that binds to the 5HT-2A receptor at a nM below XX is hereby considered a Schedule I compound"
i mean, bans like that are already happening with synthetic cannabinoids, but still.... being able to quantify this legal gray area we enjoy might not be such a blessing just yet.
SeenSoFar
Bluelighter
- Joined
- Jun 21, 2013
- Messages
- 241
Once we're that advanced machines that will synth anything from basic ingredients + power shouldn't be far off
So instead of porn you'd be downloading drugs.
Yeah, I believe in science.
This is a lot closer than you might think. I was reading an article the other day where someone was conjecturing that, within the next decade or two, 3D printers will become available to print on an atomic scale. It will be so fascinating when it progresses to the point that you load a print cartridge of carbon, hydrogen, nitrogen, and oxygen into your printer, along with any other raw atomic material you need, load in a ChemDraw file, specify the molar weight to be synthesized, and click print. I can imagine that such technology will render current drug laws obsolete, since anyone with access to such a device will be able to create whatever they desire.
Its actually a bit of a frightening prospect. Some teenager with little to no chemical knowledge starts loading things from this thread into his molecular printer and then doses them to see what happens. What a brave new world...
ShaggyFin
Bluelighter
- Joined
- Jan 15, 2013
- Messages
- 751
Could Beta Ketones be the future?
I have read somewhere that you can add an "aromatic" beta ketone compound to tons of different terpenes and other alkaloids using acetone, and I was wondering if anyone had any more information on that.
Acetone is also called "Beta-Ketopropane"
http://en.wikipedia.org/wiki/Beta-ketopropane
And it has sisters.
So even if acetone can't do it, maybe one of these can.
http://en.wikipedia.org/wiki/Ketone_bodies
http://en.wikipedia.org/wiki/3-hydroxybutanal
http://en.wikipedia.org/wiki/Mannich_base
http://en.wikipedia.org/wiki/Damascone
The three endogenous ketone bodies are acetone, acetoacetic acid, and beta-hydroxybutyric acid.[4] Other ketone bodies such as beta-ketopentanoate and beta-hydroxypentanoate may be created as a result of the metabolism of synthetic triglycerides such as triheptanoin
I know there are things like bk-MDMA, bk-MBDB, etc in existence. Is there a possibility of something like a bk-DMT or bk-DXM or bk-THC bk-Etizolam bk-Mescaline bk-JWH bk-Kavalactones bk-Cocaine bk-PEA (alpha-ethyl PEA is active, so why not)?
If you read here this guy says bk-DET exists, but I can't find an example online.
http://www.bluelight.org/vb/threads/...=1#post5146876
I also found a PDF book online that has one reference to a 5-MeO-bk-DiPT
And this
http://www.bluelight.org/vb/threads...methylenedioxy-group-to-an-ephedrine-molecule
Beta-Meo-MDMA
http://www.bluelight.org/vb/threads/686964-MeO-MDMA
And this
http://www.bluelight.org/vb/threads...enzofurans?p=12195710&viewfull=1#post12195710

Also this
http://en.wikipedia.org/wiki/Aldol_adduct
http://en.wikipedia.org/wiki/Ketogenesis
http://www.bluelight.org/vb/threads/357814-Beta-keto-amphetamines-(cathinones)-amp-stability
http://www.bluelight.org/vb/threads/306522-alpha-keto-tryptamines-3-acetamideindoles
http://www.bluelight.org/vb/threads...enethylamines-Beta-MeO-PEA-s-BM-PEA-s-(BMP-s)
http://www.bluelight.org/vb/threads/688503-Alpha-Ethyl-Psychedelic-Amphetamines-(Aephetamines)
AMT is really just a-MT or alpha-MethylTryptamine. So there is probably a bk- and a- for everything, and I think they are available through Aldol reaction methods.
bk-2c-B exists... Why not bk-2c-I, or bk-25I-NBOMe, bk-MDA, bk-Mescaline, etc?
I have read somewhere that you can add an "aromatic" beta ketone compound to tons of different terpenes and other alkaloids using acetone, and I was wondering if anyone had any more information on that.
Acetone is also called "Beta-Ketopropane"
http://en.wikipedia.org/wiki/Beta-ketopropane
And it has sisters.
So even if acetone can't do it, maybe one of these can.
http://en.wikipedia.org/wiki/Ketone_bodies
http://en.wikipedia.org/wiki/3-hydroxybutanal
http://en.wikipedia.org/wiki/Mannich_base
http://en.wikipedia.org/wiki/Damascone
The three endogenous ketone bodies are acetone, acetoacetic acid, and beta-hydroxybutyric acid.[4] Other ketone bodies such as beta-ketopentanoate and beta-hydroxypentanoate may be created as a result of the metabolism of synthetic triglycerides such as triheptanoin
I know there are things like bk-MDMA, bk-MBDB, etc in existence. Is there a possibility of something like a bk-DMT or bk-DXM or bk-THC bk-Etizolam bk-Mescaline bk-JWH bk-Kavalactones bk-Cocaine bk-PEA (alpha-ethyl PEA is active, so why not)?
If you read here this guy says bk-DET exists, but I can't find an example online.
http://www.bluelight.org/vb/threads/...=1#post5146876
I also found a PDF book online that has one reference to a 5-MeO-bk-DiPT
And this
http://www.bluelight.org/vb/threads...methylenedioxy-group-to-an-ephedrine-molecule
Beta-Meo-MDMA
http://www.bluelight.org/vb/threads/686964-MeO-MDMA
And this
http://www.bluelight.org/vb/threads...enzofurans?p=12195710&viewfull=1#post12195710

Also this
http://en.wikipedia.org/wiki/Aldol_adduct
http://en.wikipedia.org/wiki/Ketogenesis
http://www.bluelight.org/vb/threads/357814-Beta-keto-amphetamines-(cathinones)-amp-stability
http://www.bluelight.org/vb/threads/306522-alpha-keto-tryptamines-3-acetamideindoles
http://www.bluelight.org/vb/threads...enethylamines-Beta-MeO-PEA-s-BM-PEA-s-(BMP-s)
http://www.bluelight.org/vb/threads/688503-Alpha-Ethyl-Psychedelic-Amphetamines-(Aephetamines)
AMT is really just a-MT or alpha-MethylTryptamine. So there is probably a bk- and a- for everything, and I think they are available through Aldol reaction methods.
bk-2c-B exists... Why not bk-2c-I, or bk-25I-NBOMe, bk-MDA, bk-Mescaline, etc?
Last edited by a moderator:
sekio
Bluelight Crew
I have read somewhere that you can add an "aromatic" beta ketone compound to tons of different terpenes and other alkaloids using acetone, and I was wondering if anyone had any more information on that.
Acetone will react in e.g. aldol condensations, but no it can't be used to just "add a beta ketone", nor can any of those other compounds listed. Aldol condensations are something totally different, they form carbon-carbon bonds.
I know there are things like bk-MDMA, bk-MBDB, etc in existence. Is there a possibility of something like a bk-DMT or bk-DXM or bk-THC bk-Etizolam bk-Mescaline bk-JWH bk-Kavalactones bk-Cocaine?
Considering many of those aren't phenethylamines/tryptamines, no, there can't be "BK-cocaine". All that the term means is there's a ketone on the second carbon away from the amine in a PEA/tryptamine.
The above image of bk-2C-B-NBOMe is missing the letter "r" next to the "B". Br stands for bromine / colliquially bromo (which is the prefix name for the element bromine), B stands for boron which is another element.
bk-MDA, if at all active, would probably need a ridiculous dose like something along the lines of 400 mg if there is ~4x potency loss when beta-ketonating 2C-B. I really don't think it is a smart idea to put that kind of strain on your vitals.
bk-Mescaline even worse, a dose may be over a gram, though it wouldn't be a subbed cathinone like bk-MDA.
The term beta ketone refers to putting that carbonyl oxygen on the beta position. The term beta position (wiki search substituted phenethylamine to find out what it is) is only meaningful in a limited context*. So on terpenes you can certainly 'design' an additional =O carbonyl oxygen, but that doesn't magically create an interesting structure. It only makes sense if you depart from a certain drug that would benefit from the modification.
bk-2C-B may have its fans and the prospect of other bk-2C-X compounds can be fascinating as long as the starting material is not too weak to begin with (which would yield the problems I listed at the start of this post), but that may mostly be by the virtue of cathinone (again look up the structure) being an active compound itself. So there may be some crossover pharmacodynamics - which often don't work well by the way - and quite frankly the dimerisation problem giving inconsistent responses to dosage and the general impotency are a pain in the ass. These problems (not sure which exactly) seem to be causing the drop in potency.
Also prepare to be told by people that cathinones are categorically shitty.
If you like methylone then obviously beta-ketone versions of the MAPBs / MAPDB should be fun, yes I would expect those surfacing first and foremost. Those actually make sense and don't go so far out on a limb.
No offense, I applaud the enthousiasm, but you need to either learn some more chemistry and/or stay more close to home regarding drug design. Ketonating the shit out of random chemicals does not mean anything per se, it is a trivial thing in organic chem and there are other considerations if it's going to be worth investigating any bit.*
(What Sekio said plus where I marked * are reasons why bk-etizolam, bk-kavalactones, etc don't make sense. With some exceptions perhaps, for example 'beta-keto tryptamines' apparently have been discussed, just BL-google that)
bk-MDA, if at all active, would probably need a ridiculous dose like something along the lines of 400 mg if there is ~4x potency loss when beta-ketonating 2C-B. I really don't think it is a smart idea to put that kind of strain on your vitals.
bk-Mescaline even worse, a dose may be over a gram, though it wouldn't be a subbed cathinone like bk-MDA.
The term beta ketone refers to putting that carbonyl oxygen on the beta position. The term beta position (wiki search substituted phenethylamine to find out what it is) is only meaningful in a limited context*. So on terpenes you can certainly 'design' an additional =O carbonyl oxygen, but that doesn't magically create an interesting structure. It only makes sense if you depart from a certain drug that would benefit from the modification.
bk-2C-B may have its fans and the prospect of other bk-2C-X compounds can be fascinating as long as the starting material is not too weak to begin with (which would yield the problems I listed at the start of this post), but that may mostly be by the virtue of cathinone (again look up the structure) being an active compound itself. So there may be some crossover pharmacodynamics - which often don't work well by the way - and quite frankly the dimerisation problem giving inconsistent responses to dosage and the general impotency are a pain in the ass. These problems (not sure which exactly) seem to be causing the drop in potency.
Also prepare to be told by people that cathinones are categorically shitty.
If you like methylone then obviously beta-ketone versions of the MAPBs / MAPDB should be fun, yes I would expect those surfacing first and foremost. Those actually make sense and don't go so far out on a limb.
No offense, I applaud the enthousiasm, but you need to either learn some more chemistry and/or stay more close to home regarding drug design. Ketonating the shit out of random chemicals does not mean anything per se, it is a trivial thing in organic chem and there are other considerations if it's going to be worth investigating any bit.*
(What Sekio said plus where I marked * are reasons why bk-etizolam, bk-kavalactones, etc don't make sense. With some exceptions perhaps, for example 'beta-keto tryptamines' apparently have been discussed, just BL-google that)
Last edited:
ShaggyFin
Bluelighter
- Joined
- Jan 15, 2013
- Messages
- 751
A New Class of Drugs
This idea started in this thread
http://www.bluelight.org/vb/threads/716103-Could-Beta-Ketones-be-the-future
http://en.wikipedia.org/wiki/Damascone
It's in Roses, and it's already a Cyclohexyl and Beta Ketone.

OR
http://en.wikipedia.org/wiki/Myrcene
A strange maybe "ready" molecule.

Now, looking at those and these.
http://en.wikipedia.org/wiki/Acetone
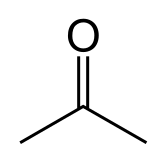
http://en.wikipedia.org/wiki/Chloroform

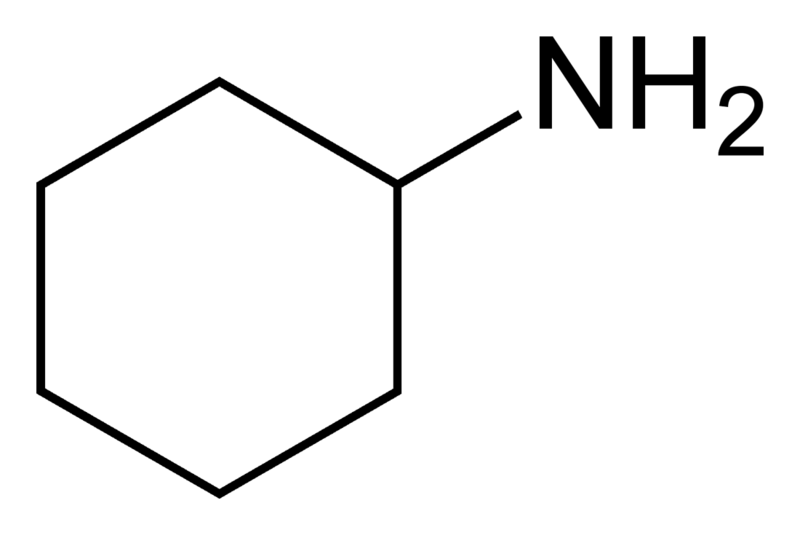
http://en.wikipedia.org/wiki/Cyclohexylamine
http://en.wikipedia.org/wiki/Nitroethane

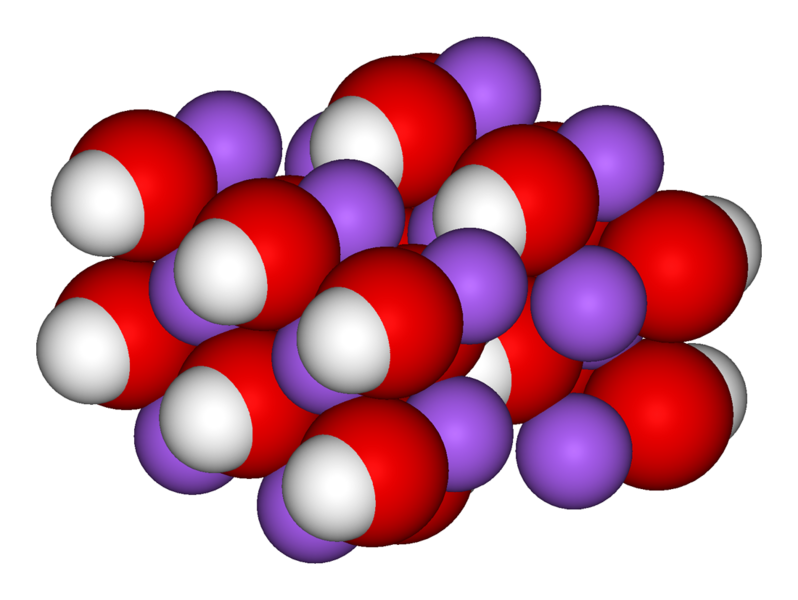
http://en.wikipedia.org/wiki/Naoh
I feel like something is here...
This idea started in this thread
http://www.bluelight.org/vb/threads/716103-Could-Beta-Ketones-be-the-future
http://en.wikipedia.org/wiki/Damascone
It's in Roses, and it's already a Cyclohexyl and Beta Ketone.

OR
http://en.wikipedia.org/wiki/Myrcene
A strange maybe "ready" molecule.

Now, looking at those and these.
http://en.wikipedia.org/wiki/Acetone

http://en.wikipedia.org/wiki/Chloroform

http://en.wikipedia.org/wiki/Cyclohexylamine

http://en.wikipedia.org/wiki/Nitroethane

http://en.wikipedia.org/wiki/Naoh

I feel like something is here...
Last edited by a moderator:
- Status
- Not open for further replies.




































