-
Neuroscience & Pharmacology Discussion Welcome Guest
Posting Rules Bluelight Rules Recent Journal Articles Chemistry Mega-Thread FREE Chemistry Databases! Self-Education Guide -
N&PD Moderators: Skorpio | someguyontheinternet
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
I Like to Draw Pictures of Random Molecules
- Thread starter nuke
- Start date
- Status
- Not open for further replies.
[MENTION=387185]DotChem[/MENTION], ^ tried that one p-I-BZP plain weak stimulant with heart-racing effect on crash
[MENTION=99760]Nagelfar[/MENTION], (several structures above), i still see alot of "formaldehyde" on many cociane's analogue posted up there, (methyleneimine moeity!) also some N-N-N silgle bond in a ring seems to explode into nitrogen gas.
[MENTION=99760]Nagelfar[/MENTION], (several structures above), i still see alot of "formaldehyde" on many cociane's analogue posted up there, (methyleneimine moeity!) also some N-N-N silgle bond in a ring seems to explode into nitrogen gas.
Nagelfar
Bluelight Crew
[MENTION=99760]Nagelfar[/MENTION], (several structures above), i still see alot of "formaldehyde" on many cociane's analogue posted up there, (methyleneimine moeity!) also some N-N-N silgle bond in a ring seems to explode into nitrogen gas.
To be 100% honest; my only two completely serious attempts were the first and last structure I posted in my first post on this page (pg. 142) of this thread.
However, looking into the remote binding cite/site and the viability of such in phenyltropanes and cocaine analogues I think:
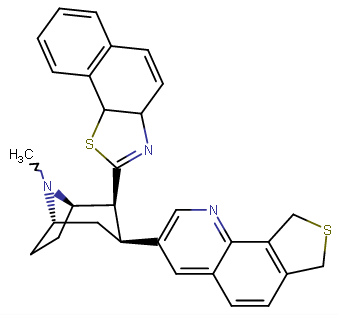
^I call it "Epibatinaphthygnan" cf. RTI-470 for the C2 + the "3-(2-thiophene) and 3-(2-furan)" class, then for the C3 the epibati tropane, the naphthyl, and tamagnan. Then notice the overall configuration of where the cocaine esters would be and how they were mirrored in one another is again repeated in this compound, but for different reasons and apparent optimization.
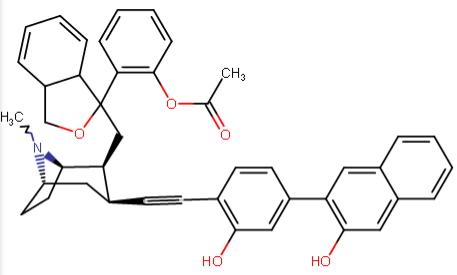
^a non-phenyltropane cocaine analogue keeping same length (with steric differences) spacer as benzoyloxy class analogues but with additions to para pos. to attempt to incorporate putative "remote ligand site @ DAT" binding affinity and C2 citalopram inspired branch.
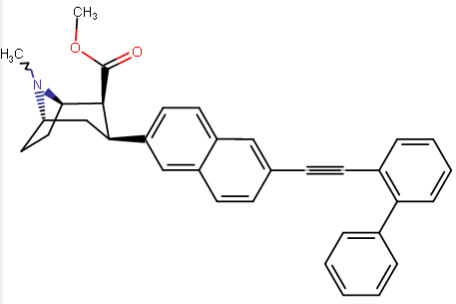
^naphthyl-phenyltropane partial-inversion of the one above @ C3
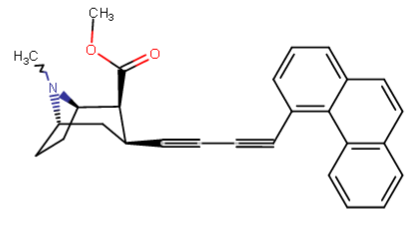
^Anyhow... these are my recent, serious, inspirations from the ten new para substituted phenyltropanes that are added to the WP 'List of Phenyltropanes' along with my usual predilections to make more cocaine analogues proper besides 'phenyltropanes' but always about as many PTs as well. etc. ;-p
Looks much nicer, good work Nagelfar!
I think i have drawn somewhere similar structure to the second picture, except not naphthyl but phenyl, and the two phenolic OH are OMe in my design. i havnt paid much interest in it much tho (bcos i was then into drawing alot of cannabinoids ligands)
The last one tho, can be made pure but idk how much to expect for shelflife lifetime.
(See: Polydiacetylene))
I think i have drawn somewhere similar structure to the second picture, except not naphthyl but phenyl, and the two phenolic OH are OMe in my design. i havnt paid much interest in it much tho (bcos i was then into drawing alot of cannabinoids ligands)
The last one tho, can be made pure but idk how much to expect for shelflife lifetime.
(See: Polydiacetylene))
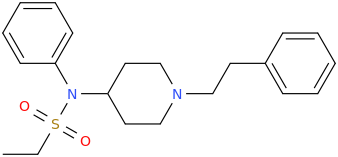
@DotChem, ^ tried that one p-I-BZP plain weak stimulant with heart-racing effect on crash
How does it compare to MDMA though? actually the idea behind replacing the phenyl of BZP with 4-iodophenyl is to make BZP less stim and more empathogen ie.more MDMA-like by increasing serotonergic DRA activity of BZP: (notwithstanding escaping legality issues!!
Ratios of DA:NE:SERT releasing activity of BZP is ~ 1:1:10 (ie 10x less active as SER than DA or NE)
Ratios of DA:NE:SERT of MDMA is ~ 1:1:0.1 (ie the opposite 10x more Serotonergic than dopaminergic/NE-ergic)
On the other hand, hydrophobic substituents increase the serotonergic ratio of RAs/RIs (relative to DA/NE) as in for example:
going from amphetamine to PAL-287 (Naphthylisopropylamine) changing a phenyl with more lipophilic naphthyl:
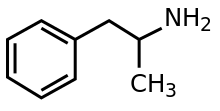
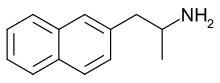
https://en.wikipedia.org/wiki/NaphthylaminopropaneNaphthylisopropylamine (PAL-287) is an experimental drug currently under investigation for the treatment of alcohol and stimulant addiction.[1]
Naphthylisopropylamine acts as a non-neurotoxic[2] releasing agent of serotonin, norepinephrine, and dopamine, with EC50 values of 3.4 nM, 11.1 nM, and 12.6 nM, respectively.[3] It also has affinity for the 5-HT2A, 5-HT2B, and 5-HT2C receptors (EC50 values = 466 nM, 40 nM, and 2.3 nM, respectively),[1] ...In animal studies, naphthylisopropylamine was shown to reduce cocaine self-administration, yet produced relatively weak stimulant effects when administered alone, being a (much) lesser stimulant than d-amphetamine for comparison...and blabla..
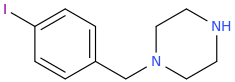
So the idea is to design piperazines MDMA substitutes. The 4-iodophenyl doesn't seem to work according to your experience. so may be naphthylmethylpiperazine where naphthyl replace BZP phenyl like this:
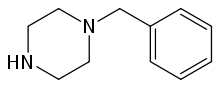
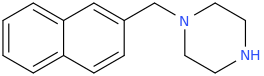
(disclaimer: piperazines are illegal in some countries including the US, UK DE AU CA ..etc
Nagelfar
Bluelight Crew
Looks much nicer, good work Nagelfar!
I think i have drawn somewhere similar structure to the second picture, except not naphthyl but phenyl, and the two phenolic OH are OMe in my design. i havnt paid much interest in it much tho (bcos i was then into drawing alot of cannabinoids ligands)
Interesting you had methoxy and not hydroxy for the bi-phenyl when you were *more interested in cannabinoids* at the time, seeing as there are many cannabinoids closer to the structure I gave, ala: ''cannabicyclohexanol''. In fact, it wasn't my rationale for making it, but I thought about it as I uploaded the image here that mine had some cannabinoid-related structure.
MD-BZP(or rather its prodrug Fipexide) is an antidepressant marketed as antidepressant/wakefulness/nootropic in italy france and germany in the 80s. MD-BZP was later shown to be the "active ingredient". I guess it is more like typical SSRIs, with no stim activity. Who knows how the naphthyl analog will behave: more like MDMA or SSRIs? hard to tell. (worth the try tho of the naphthyl-BZP as MDMA substituted ..pretty straightforward to synthesize dirt cheap).I don't know a whole lot about piperazines, but I do recall hearing that
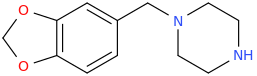
1-PIPERONYL-PIPERAZINE
isn't, subjectively, very similar to MDMA at all.

If the hypothesis that says increase lipophilicity increase serotonergic vs. DAT/NET of piperazines, then the methylenedioxy anallog won't be more similar to MDMA as expected but a shitty stim (BZP is only 1/10 AMPH!).
It is even less lipophilic (logP=1.00) than the parent BZP(logP=1.38 . The naphthyl-Piperazine seems way much better (logP = 2.37). Worth the try!
https://en.wikipedia.org/wiki/Fipexide
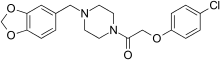
[MENTION=387185]DotChem[/MENTION]
Phenyl/benzylpiperazine has different SAR to PEA-class of compound,
you cannot just apply the functional group and expecting results in the same way.
I supposed Benzylpiperazines follow more closely to cocaine-type SAR, try looking at piperazine ring as part of the 6-membered ring in that.
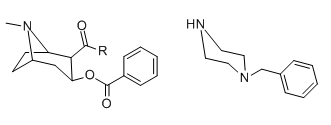
Where Phenylpiperazines are unselective agonist at 5HT receptor, altering electron density on that aromatic ring u get differing ratio between subtypes, but they are still so very unselective.
[MENTION=99760]Nagelfar[/MENTION]
I dismissed it as being CB receptor ligand as soon as i saw that, it size and shape/direction (where each groups should point to) cant seem to fit in the pocket. I also care somewhat less about the group, as CBr seems to tolerate fn grp changes quite alot as long as u have correct requirements and spacing between em. (eg, two hydrophobic patch 1-3 atoms split apart, better if one has pi bond, and a long alkyl tail (which can also bulge or puckered at the end also))
One that i drew was like having biphenyl replacing your phenyl-naphthyl and having varying amount/positions of -OMe and -OCH2O- on em. The idea was from salicylyl replacing the benzoyl.
Phenyl/benzylpiperazine has different SAR to PEA-class of compound,
you cannot just apply the functional group and expecting results in the same way.
I supposed Benzylpiperazines follow more closely to cocaine-type SAR, try looking at piperazine ring as part of the 6-membered ring in that.

Where Phenylpiperazines are unselective agonist at 5HT receptor, altering electron density on that aromatic ring u get differing ratio between subtypes, but they are still so very unselective.
[MENTION=99760]Nagelfar[/MENTION]
I dismissed it as being CB receptor ligand as soon as i saw that, it size and shape/direction (where each groups should point to) cant seem to fit in the pocket. I also care somewhat less about the group, as CBr seems to tolerate fn grp changes quite alot as long as u have correct requirements and spacing between em. (eg, two hydrophobic patch 1-3 atoms split apart, better if one has pi bond, and a long alkyl tail (which can also bulge or puckered at the end also))
One that i drew was like having biphenyl replacing your phenyl-naphthyl and having varying amount/positions of -OMe and -OCH2O- on em. The idea was from salicylyl replacing the benzoyl.
Last edited:
Nagelfar
Bluelight Crew
One that i drew was like having biphenyl replacing your phenyl-naphthyl and having varying amount/positions of -OMe and -OCH2O- on em. The idea was from salicylyl replacing the benzoyl.
I'm really interested in the naphthyl-PT substitutions: Can't wait until I get this beyond just its abstract, I'm going to have a heyday:
Synthesis of 2β-Acyl-3β-(substituted naphthyl)-8-azabicyclo[3.2.1]octanes and Their Binding Affinities at Dopamine and Serotonin Transport Sites

adder
Bluelighter
- Joined
- Mar 28, 2006
- Messages
- 2,851
Perhaps someone should make some use of this infinitive number of phenyltropanes and not just synthesize more and more for whatever reason. On the other hand I'd gladly see such a big number of modified morphinans, I know there are a lot of them, but still, the possibilities are endless
Nagelfar
Bluelight Crew
Perhaps someone should make some use of this infinitive number of phenyltropanes and not just synthesize more and more for whatever reason. On the other hand I'd gladly see such a big number of modified morphinans, I know there are a lot of them, but still, the possibilities are endless
Yeah, but, none of the extant PTs show the potential they could if you've delved into the QSAR they show for as long as I have.
For example, you have π–π stacking possibilities includ. T-shaped and parallel-displaced on the secondary benzenes on the C3 phenyl that haven't even been looked into on the primary/singular; which if is an issue of steric factors, may do better than the sandwiched kind of the same coordination complex. There's just too many good avenues that haven't been exploited yet.
Last edited:
Nagelfar
Bluelight Crew
my 3C-PEP closed/open DAT binding phenyltropane / benztropine comparison post from other thread.
^the above inspired me to make:
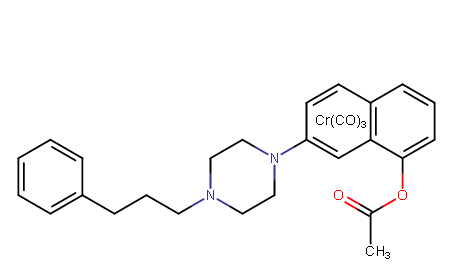
Perhaps the increase to the phenylethyl by one methylene unit would give it a NET/DAT ratio closer to vanoxerine? Don't see why the naphthyl, as univerally seems to at that carbon length, wouldn't dramatically potentiate its DAT affinity; and furthermore gave the first benzene of the naphthyl the chromium coordination complex that doubled the strength of PTs in similar positioning when so chelated, and gave the 2nd benzene of the naphthyl the acetoxy that would be in the same place as ortho-acetoxy-cocaine that not only potentated the latter's affinity, but greatly enhanced its speed of onset across the BBB as well (and natural de-acetylation leaving it a phenol increases potency even more after entering the BBB, but doesn't have same speed of onset prior to de-acetylation).
^the above inspired me to make:

Perhaps the increase to the phenylethyl by one methylene unit would give it a NET/DAT ratio closer to vanoxerine? Don't see why the naphthyl, as univerally seems to at that carbon length, wouldn't dramatically potentiate its DAT affinity; and furthermore gave the first benzene of the naphthyl the chromium coordination complex that doubled the strength of PTs in similar positioning when so chelated, and gave the 2nd benzene of the naphthyl the acetoxy that would be in the same place as ortho-acetoxy-cocaine that not only potentated the latter's affinity, but greatly enhanced its speed of onset across the BBB as well (and natural de-acetylation leaving it a phenol increases potency even more after entering the BBB, but doesn't have same speed of onset prior to de-acetylation).
You talking about 3C-PEP? yeah of course it is not really a phenylpiperazine nor a PEA. Its SAR (depending on SAR of what effect you talking about?) may or may not be similar. But 3C-PEP potential metabolite (3-Chlorophenyl) piperazine mCPP is a phenylpiperazine. Is that what you talking about?@DotChem
Phenyl/benzylpiperazine has different SAR to PEA-class of compound,
Where did I do that? .. have no idea what you talking about?@DotChem
you cannot just apply the functional group and expecting results in the same way.
my 3C-PEP closed/open DAT binding phenyltropane / benztropine comparison post from other thread.
^the above inspired me to make:

Perhaps the increase to the phenylethyl by one methylene unit would give it a NET/DAT ratio closer to vanoxerine? Don't see why the naphthyl, as univerally seems to at that carbon length, wouldn't dramatically potentiate its DAT affinity; and furthermore gave the first benzene of the naphthyl the chromium coordination complex that doubled the strength of PTs in similar positioning when so chelated, and gave the 2nd benzene of the naphthyl the acetoxy that would be in the same place as ortho-acetoxy-cocaine that not only potentated the latter's affinity, but greatly enhanced its speed of onset across the BBB as well (and natural de-acetylation leaving it a phenol increases potency even more after entering the BBB, but doesn't have same speed of onset prior to de-acetylation).
@Nafelgar: increasing the phenethyl chain by one carbon completely and utterly destroy DAT activity of 3C-PEP:
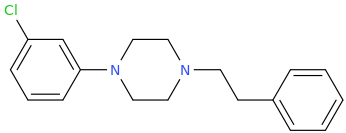
Ki @ DAT = 0.04nM
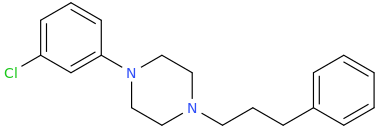
Ki @ DAT >10,000nM
That is: the phenylpropyl is at least 250,000 less potent than the Phenylethyl!!
That actually points toward a DAT binding site of this class different from that of large, highly lipophilic vanexorine-like DRI piperazines. It may not behave like cocaine as stim tho, since its lacking NET/SERT activity and some of cocaine stim due to NET inhibiton. At least at lower doses, enough for dopaminergic activation, you won't see any NET stimulation.
IMHO, 3C-PEP would propably be a pure DRI stim like amfonelic acid, without antibiotic problem associated with AFA but with much of AFA extremely reinforcing effect. For one, it'll potentially be a potent aphrodisiac and nootropic like AFA.. but who knows? It looks pretty straighforward to synthesize very very cheaply! (no synthesis talk here but just saying. It may show up on the market pretty soon (as nootropic maybe??) if vendors paying attention. Would be nice to have a review of this compound..
Nagelfar
Bluelight Crew
@Nafelgar:
*Ahem* (you butchered my nom de guerre ;-j) (Nagel-far (Gmc. "Nagel" = "Nail", gutteral proto-Germanic "G" rather being between a y & g as per the "yogh" character i.e. "Naȝl" + Gmc. 'far' = D. "fahr" = (far-afield / travelling) = Eng. 'ferry', e.g. "ferried across" = traversing by (finger)-nail. Typing via internet around world, etc.)
increasing the phenethyl chain by one carbon completely and utterly destroy DAT activity of 3C-PEP:

Ki @ DAT = 0.04nM

Ki @ DAT >10,000nM
That is: the phenylpropyl is at least 250,000 less potent than the Phenylethyl!!
That IMHO only reinforces why the beta-configured benztropines do better in alpha, and the mono-aryl phenyltropanes do better in beta orientation @ C3 with the same binding site and I'm willing to bet it is the same even....
That actually points toward a DAT binding site of this class different from that of large, highly lipophilic vanexorine-like DRI piperazines.
...with 3C-PEP
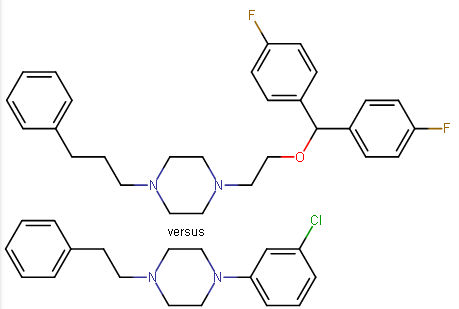
So I'd have to respectfully disagree along with my mention above, it's all in the di-aryl configuration fitting into the binding site and having several carbons more length at one end, if the meta-chlorotoluene on the 3C-PEP were altered to, say a m-chloropropylbenzene, my guess would be that it could rotate to fit the binding site more neatly, even with the phenylpropyl chosen in place of the phenylethyl: check out the major differences in the GBRs in this case (vanexorine analogs)
e.g.
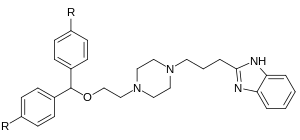
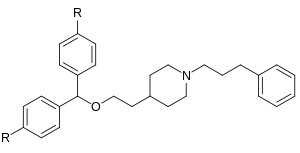
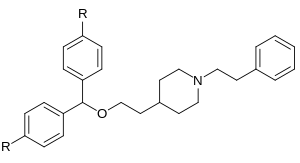
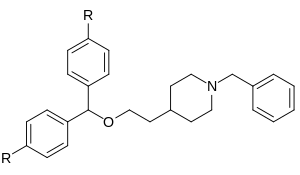
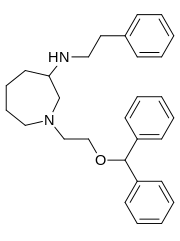
Whether its still not compromised, well, we may need another parent privileged scaffold to get more specific in this direction.
Last edited:
crmt28
Bluelighter
- Joined
- Jun 19, 2014
- Messages
- 52
This was probably already posted here, but can we make any changes to the cyclohexane ring in arylcyclohexylamines without losing activity?
I know changing the ring size (cylcopentyl, cycloheptyl) makes it totally inactive, but what about substitutions?
A few examples:
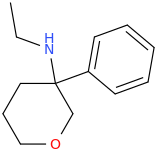
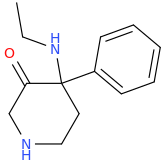
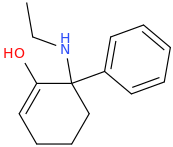
Silly bonus molecule:
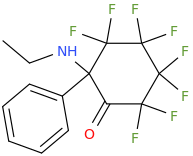
I know changing the ring size (cylcopentyl, cycloheptyl) makes it totally inactive, but what about substitutions?
A few examples:



Silly bonus molecule:

Sorry [MENTION=99760]Nagelfar[/MENTION]! reason I got that upside down was I had the naphthyl tropane you drew in mind when I posted that. Naph you know as in Naf?!. My german is limited (to organic chemistry german as in how to synthesize Naphthaline-TropanSaure Esters*Ahem* (you butchered my nom de guerre ;-j) (Nagel-far (Gmc. "Nagel" = "Nail", gutteral proto-Germanic "G" rather being between a y & g as per the "yogh" character i.e. "Naȝl" + Gmc. 'far' = D. "fahr" = (far-afield / travelling) = Eng. 'ferry', e.g. "ferried across" = traversing by (finger)-nail. Typing via internet around world, etc.)..
That IMHO only reinforces why the beta-configured benztropines do better in alpha, and the mono-aryl phenyltropanes do better in beta orientation @ C3 with the same binding site and I'm willing to bet it is the same even....
...with 3C-PEP

So I'd have to respectfully disagree along with my mention above, it's all in the di-aryl configuration fitting into the binding site and having several carbons more length at one end, if the meta-chlorotoluene on the 3C-PEP were altered to, say a m-chloropropylbenzene, my guess would be that it could rotate to fit the binding site more neatly, even with the phenylpropyl chosen in place of the phenylethyl: check out the major differences in the GBRs in this case (vanexorine analogs)
Of course, yeah you can force it to adapt to GBRs binding site by appending extended phenyl alkyl in place of the chlorophenyl. That's not the issue with 3C-PEP in my opinion. Question really is: does it bind (and trigger same conformational changes and downstream physiological effect as GBR?
Whether its still not compromised, well, we may need another parent privileged scaffold to get more specific in this direction.
Actually the piperazine scaffold is a very common widely used scaffold used to design anything from vaginal cream antifungal drugs to antihistamine cold meds. Keep i mind also the issue of alfa and beta anomer (with the tropane and piperidines or cyclhexyl for that matter) is not there with piperazine because of the nitrogen inversion: (google it). So the 3-chlorophenyl is really positioned anywhere in betwenn the axial (alfa anomer) and the equatorial (the beta anomer). basically half way between alfa and beta. Plus, that nitrogen is also in the same plane as the phenyl because of resonnance conjugation . So the conformation of 3C-PEP piperazine is rather different. Actually you'll be surprised that the energy minimized conformation of 3C-PEP overlay with that of methylphenidate or even PVP..
- Status
- Not open for further replies.