ShaggyFin
Bluelighter
- Joined
- Jan 15, 2013
- Messages
- 751
If you have not read PIHKAL over and over and over in actual book form, then you may not have noticed this.
Sasha mentions the Magical Half Dozen, which is 2C-I, etc
He mentions the 10 Classic Ladies, which is Florence, Ganesha, etc.
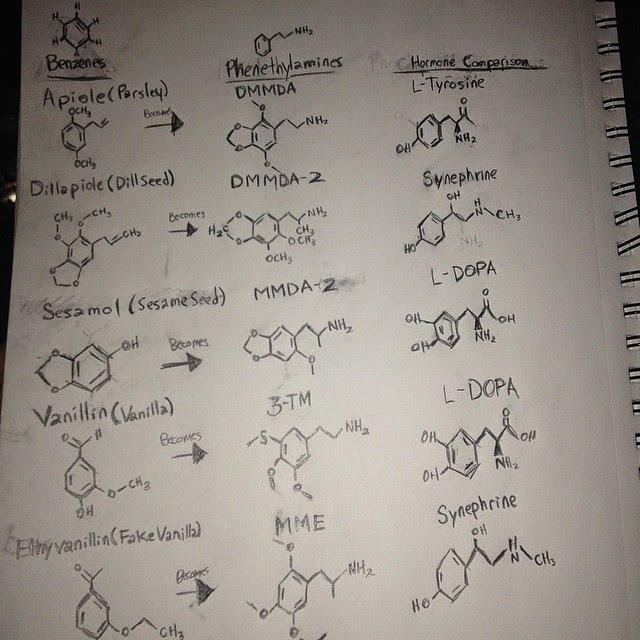
But he also mentions that it may be possible to create Molecules using your own liver by turning different enzymes on and off. Then on one of the recipes he gives you a list of all the Phenethylmaines that can be found in your Kitchen's Spice Cabinet, and then throughout PIHKAL he gives you the recipes for each of those Phenethylamines. He says that they just need to be Aminized, either in your liver or in a lab, and they will become active. This theory has been proven by what is now called Oilahuasca. If you mix different things together you can get different effects from different Molecules, it is based on the idea of Pro-Drugs and CYP450 Enzymes (Similar to MAOs which are effected by MAOIs). Some people have reported Psychedelic effects from Mixing just Coffee, Almond, Cinnamon, Vanilla and Nutmeg.
This is similar to how Saffrole, from Sassafras, can be used to make MDA or Ecstasy.
From the person who Discovered Oilahuasca
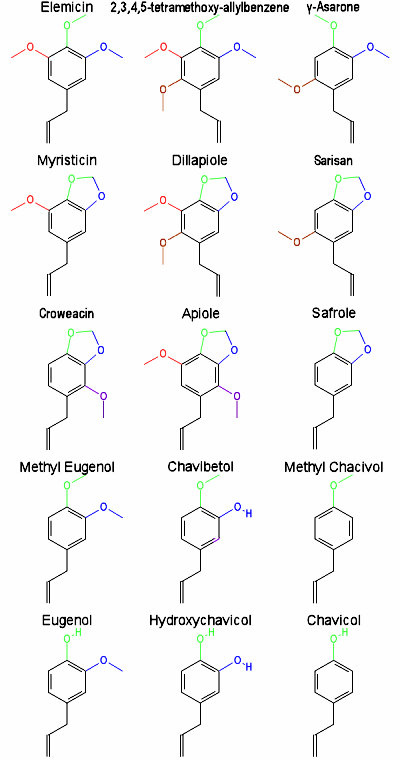
The benzene ring has 6 positions. In the graphical layout given below the following color guide is used:
position 1 = black (the important allyl side chain, required for conversion to an alkaloid)
position 2 = brown (a methoxy group here seems to cause LSD-like mental effects)
position 3 = red
position 4 = green (must be a methoxy or methylenedioxy group for psychedelic activity)
position 5 = blue (a methoxy or methylenedioxy group here seems to enhance visuals)
position 6 = purple (a methoxy group here probably adds speedy effects)
Position 1 has the allyl side chain hanging off of it. It's the same for all allylbenzenes. This is the part of the allylbenzene that reacts in the body to form a dimethylamine, piperidine, or pyrrolidine alkaloid, if digested properly. The details of this are discussed elsewhere.
In order to have psychedelic activity, position 4 must be a methoxy group. It can be tied to another methoxy group on position 5, as it is with myristicin and others. Two methoxy groups tied together are called a methylenedioxy group.
Position 4 cannot be a hydroxy group as it is in eugenol, hydroxychavicol, and chavicol. This can only lead to stimulant effects, not psychedelic effects.
At the bottom of the chart you can see eugenol, hydroxychavicol, and chavicol. These posses no psychedelic activity, even when properly metabolized. These are the only allylbenzenes in the chart that have a hydroxy group on the 4 position.
Above that we have methyl eugenol, chavibitol, and methyl chavicol. Methyl chavicol and methyl eugenol have psychedelic activity when properly metabolized. The effects of chavibitol are unknown, but are probably like a cross between methyl eugenol and methyl chavicol. The 5 position being a methoxy group seems to improve visual effects.
Above that we have croweacin, apiole, and safrole. Apiole and safrole are psychedelic when metabolized properly. Croweacin is a positional isomer of myristicin. It's activity is not known. It's very likely similar to myristicin, but probably more speedy like apiole. The 6 position being a methoxy group seems to add amphetamine style speedy effects.
Above that we have the ever so popular myristicin, and then dillapiole and the rare sarisan. Both myristicin and dillapiole are psychotic when properly metabolized. The activity of sarisan is unknown. It is a positional isomer of myristicin. It has a methoxy group on the 2 position instead of the 3 position. This probably gives it LSD-like mental effects which are attributed to dillapiole and gamma-asarone rather than myristicin.
Above that we have elemicin, 2,3,4,5-tetramethoxy-allylbenzene, and gamma-asarone. Elemicin is the only one of these that's known to have psychedelic activity when properly metabolized. When properly metabolized, it's effects are similar to myristicin, but more like mescaline. It has the same positional substitutions as myristicin, only the 4 and 5 positions are not tied together. Gamma-asarone is a positional isomer of elemicin and is probably the main active psychedelic compound in calamus oil from Nepal. The methoxy group on the 2 position is probably the reason calamus oil from Nepal has LSD-like mental effects shared by dillapiole and absent from most of the other allylbenzenes. I have no idea if 2,3,4,5-tetramethoxy-allylbenzene is active or not.
For those of you that are unaware, MDMA is primarily manufactured from the safrole found in sassafras essential oil.
Many bioassays of sassafras and even pure safrole show little activity. Usually a slight sedative effect, and very mild MDMA-like effect might be felt if you’re lucky. Although a few people report almost full blown MDMA-like effects from sassafras as is, most people get no effects. This points in the direction of human metabolism altering the effects of safrole.
AFOAF once tried sassafras with a CYP1A2 inhibitor: German chamomile. This experiment produced stimulant effects unlike the effects had from just sassafras alone. There was mild euphoria, and some possible mild visuals effects.
Safrole is primarily inactivated by conversion into 1-hydroxysafrole by CYP2A6. German chamomile primarily inhibits CYP1A2, so AFOAF was using the wrong inhibitor, but it was somewhat effective.
Cinnamon bark oil is a potent CYP2A6 inhibitor. This should theoretically prevent CYP2A6 from turning safrole into 1-hydroxysafrole.
My theory is that about 2-5 grams of cinnamon, or 5-10 drops of cinnamon essential oil will contain enough cinnamaldehyde to inhibit CYP2A6 and allow MDMA-like effects to be experienced from sassafras or sassafras oil.
This is only a theory. As of yet AFOAF has not tried this. He will try this very soon. Incidentally, it is the 1-hydroxysafrole which is primarily formed by CYP2A6 which is considered weakly carcinogenic and not safrole itself. So by using cinnamon oil, the weak potential carcinogenicity of safrole should be greatly reduced.
For his test he plans on using German chamomile oil at 3 drops along with the cinnamon bark oil at 6 drops. He will use sassafras bark, freshly ground, extracted into milk with lecithin, just like how you make kava. This is the best way to extract the safrole without using a solvent. He will take the sassafras milk and the oils at the same time. Hopefully this produces an MDMA-like effect. We will see.
Here's a picture of what the CYP2A6 enzyme in humans does to safrole. Once converted to 1-hydroxysafrole by CYP2A6, it becomes much more polar (the XLogP drops from 3 way down to 1.9!), and so it's far more difficult for it to enter the brain, meaning it should be far less psychedelic (assuming that safrole is actually psychedelic before this conversion). For maximum entry into the brain, CYP2A6 should be inhibited to prevent this conversion. It’s possible that CYP1A2 might be able to perform this very same conversion (it does so with methyl chavicol and elemicin), so it too should be inhibited.
On the right you can see MDMA for comparison purposes. See how 1-hydroxysafrole is less like MDMA than safrole is? The XLogP3 of safrole is 3, MDMA is 2.2, and 1-hydroxysafrole is 1.9, being the most polar of the three. Safrole should more easily cross the blood brain barrier than MDMA, but once attacked by CYP2A6 enzymes, safrole turns into 1-hydroxysafrole, and that should help prevent it from entering the brain.
Later...
Well it was tried a few hours ago.
AFOAF used 5 grams of sassafras bark, 1 gram of lecithin, ground to powder, then mixed with 1 cup of steaming hot milk and 2 ml of vegetable oil. This was left to sit for 2 hours and was then filtered. It was super hard to filter. Decanting would be a better idea.
He then mixed 6 drops of cinnamon bark oil and 3 drops of German chamomile oil into it. The German chamomile would not mix into it and just floated to the top. He mixed it as good as he could and drank it down.
Its been about 3 hours and he feels NICE. There's very obvious euphoria. Sense of touch is enhanced. He feels good. There's no sedation felt at all. It’s mildly psychedelic.
This seemed to work. But he doesn’t know what MDMA really feels like so he can’t compare it to MDMA. It does feel like a phenethylamine though. It’s very different from taking sassafras on its own.
This is a light dose. I think 10-20 grams of bark would be much better.
I would love to hear what others more familiar with MDMA think about this combination. It seems to have worked to produce an MDMA-like effect, but until others more familiar with MDMA test this out, we should take that statement as simply a guess.
Procedure
Procedure, in plain English:
Pepper would be made into a tea. Solids filtered out.
Then you would get some Anise Oil, B9 or Valerian Root (of Chinese Origin)
So that is your Pepperidine, and you activators. Now you need your Enzyme Inhibitors. You can add L-Lysine, but it is not necessary.
Vanilla and Cinnamon work, pick one or both. You also need the Aldehyde structure from one of these.
Next. German Chamomile, Cayenne Pepper Capsules or Tangerine Skin extract/capsules
Then
Almond extract, Anise Oil (if you already had it), Cinnamon, Lemon peel oil, Lime peel oil, or a cigarette or nicotine gum if you can't find anything else.
Then
CBD, Echinacea Purea, Pomegranate, Pummelo, or Calamus Oil.
Then
Clove oil, Catechin, Dill seed Oil or Goldenseal.
Then Kudzu or Glycerin or Caffeine
Not all of these things are neccisarry, but if you do 1 thing in each list, you should get very strong effects from whatever you take.
The best thing to take is Sweet Basil Extract, in it's pure form, it is known as Methyl Chavicol.
Take all that other stuff like 30 minutes to an hour before the Basil Extract, and redose the B9, Anise or Valerian root to keep the effects going without taking more.
Science
Graviola- 5-HT1a Agonist
Black Cohosh- 5-HT1A, 5-HT1D & 5-HT7 Binding
C. Foetida L.- 5-HT1A Agonist
Yokukansan- 5-HT1A Agonist
DMT hits all of these, and can be found in tons of plants.
Black Cohosh- 5-HT1D
maybe Rhodiola rosea, Albizia lebbeck & Albizia julibrissin.
5-HT1 Receptor Agonists:
Turmeric, Ginger, Ginko Bilboa, Lemon Essential Oil, Rauwolfia, Valerian, Yohimbe
Elmicin & Myristicin (in Nutmeg)- 5-HT2A Agonist
Estragole (in Sweet Basil)- 5-HT2A Agonist
Safrole (in Sassafras)- 5-HT2A Agonist
Cinnamon Bark- CYP2A6 & CYP2E1 Inhibitor (It will deplete your liver's Glutithione) Taken 1 Hour before Allybenzene,
Clove Leaf- CYP2C9, CYP3A4, CYP1A1 & CYP1B1 Inhibitor
German Chamomile- CYP1A2 Inhibitor (Caffeine may also do this)
GoldenSseal & Echinacea purpurea very effectively do the same thing.
Black seed oil, 50% EGCG, Valerian root oil, Pomegranate, Vitamin B9, 40% Ellagic extract, Rooibos 20% Gallic acid extract, Rutin, B3 & Kudzu
AllylBenzenes
Anethole, Apiol, Asarone, Carpacin, Chavibetol, Chavicol, Dillapiole, Eugenol, Isoeugenol, Isosafrole, Methyl Eugenol, Methyl Isoeugenol,
since Cinnamon is a Phenylpropanoid, and Phenylpropanoids are made from Phenelalamine, and people who took Phenelalamine claim to get better results. I decided to post a list of Phenylpropanoids also.
Caffeyl Alcohol, Cinnamaldehyde, Cinnamyl alcohol, alpha-Cyno-4-hydroxycinnamic acid, Ethyl Cinnamate, Lignin, 2,4-Methlenedioxypropiophenone, Neoflavonoids, Nordihydroguaiaretic acid, Phenylpropanoic acid, Phloretic acid, Rhododendrin & Suberin.
Star Anise Extract or B9 for CYP2C9 Induction
NMDA Receptor Plants:
Uncaria Rhynchophyllia
Psychotria Colorata
Huperzia Serrata
Most important things:
CYP2C9 Induction
Alcohol Dehydrogenase Induction
Aldehyde Dehydrogenase Inhibition
Piperidine and or Dimethylamine Supplementation
Methyl from foods
Exercise or compounds that produce effects like exercise
Less important, but still factors:
SSAO Inhibition (Caffeine, Phenethylamine, Phenelalamine, Tryptamine)
MAO-A Induction
MAO-B Induction
NDMA Antagonism
Prolactin Inhibition
Hungarian Parsley Seed is a better source of Myristicin than Nutmeg. The effects of it when activated properly are said to be like Mescaline and MDMA together. The P450 Enzymes CYP1A2 & CYP3A4 are what break this down and need to be inhibited. CYP2D6 could also play a big role.
Elmicin is something you either need Chromotography type knowledge to get, or you have to buy it in small quantities. When activated properly it is like Mescaline, when activated wrong it is like Melatonin (sleepy). CYP1A1, CYP1B1, CYP1A2, CYP2A6, CYP2C9, CYP2A6, CYP2C9 & CYP2E1 are what are needed to be inhibited to activate this. CYP2D6 could also play an important role.
Safrole is like MDMA when activated properly and like Melatonin when not. CYP2A6, CYP2C9, and CYP2E1 are most important for this. CYP2D6 could also be important.
Methyl Chavicol when activated properly is like a light speedy LSD, when activated wrong it is said to be almost like Marijuana. CYP1A2 and CYP2A6 inhibit it, and CYP2D6 could also be important.
If the CYP2D6 Enzyme is inhibited with all the others, these are possibly visually hallucinogenic Oilahuascas. And the Methyl Chavicol doesn't build a tolerance (the others do) it actually gets stronger for you every time you use it, or you can use less.
Several allylbenzenes have been proven to form up to 3 alkaloid metabolites after ingestion by several animals.[2][3] They do not form amphetamines in vivo as has been speculated in the past. The alkaloids detected in animal urine are tertiary aminopropiophenones of 3 possible subtypes: dimethylamines, piperidines, and pyrrolidines.[1][2][3][4]
The allylbenzene elemicin has been proven to form all 3 different alkaloid metabolites after ingestion in animals by analyzing urine using gas-liquid chromatography and chemical ionization mass spectrometry.[1]
Safrole is also proven to form all three alkaloid metabolites after ingestion.[2]
Myristicin appears to only form piperidines and pyrrolidines. Dimethylamines of myristicin have not been detected.[3]
Allylbenzene, from which all allylbenzenes are derived, forms piperidine and dimethylamine alkaloids.[4]
Propenylbenzene and its derivatives (asarone, anethole, etc.) do not form alkaloid metabolites.[4]
Here is how it works
CYP Enzymes (Drug Metabolism, etc)
Induction and Inhibition (Anti-Oxidants, etc)
All inhibitors of oxidative 17bHSD2 will prevent activation of allylbenzenes. This enzyme must be induced, not inhibited. It's the single most important enzyme to induce. If oxidative 17bHSD2 is not functioning, allylbenzenes cannot produce psychedelic activity. Naringenin also potently inhibits 17bHSD2. Grapefruit contains large amounts of naringenin, and also prevents the psychedelic action of allylbenzenes if taken before allylbenzenes. Inhibition lasts approximately 4-8 hours.
Oilahuasca Diet
Here's a list of all known 17bHSD2 inhibitors that should be avoided 4-8 hours prior to using allylbenzenes:
•Quercetin and all food or supplements containing large amounts of it.
•Apples (0.0263% quercetin)[6]
•Cabbage (0.01% quercetin)[6]
•Cranberry (0.025% quercetin)[6]
•Evening-Primrose (20% quercetin)[6]
•Galangin and all food or supplements containing large amounts of it.
•Garlic (0.02% quercetin)[6]
•Grapefruit (contains naringenin, kaempferol, galangin, and quercetin)
•Himalayan Mayapple (1.2% quercetin)[6]
•Kaempferide and all food or supplements containing large amounts of it.
•kaempferol and all foods that contain large amounts it.
•Mayapple (5% quercetin)[6]
•Naringenin and all food or supplements containing large amounts of it.
•Neem (0.1% Quercetin)[6]
•Oats (0.031% Quercetin)[6]
•Onions (4.81% quercetin).[6]
•Orange (4.58% naringenin)[6]
•Tea (10-25 mg/L quercetin, 6.3-17 mg/L kaempferol [7]).[6]
•Tomato (contains kaempferol, naringenin)
•Yuzu (contains naringenin)
Tasting
Sasha used to invent brand new Molecules that had never been recorded before, and then he would taste them to discover their effects.
Here is how tasting works.
He would invent a new Molecule, similar in structure to an existing molecule. And he would use different tests to see what the Molecule looked like, for example he had a machine that could tell him a few things, and if you put bromide on a molecule and it turns to blood colored or white you can tell it has different things attached to it.
Then, he would take this new Molecule, measure out 1µg and eat it.
Then over 3-4 days he would record the effects, if any, using a scale of +, ++, +++ or ++++
And the 3rd or 4th day he would take 5µg
Then 3-4 days later 10µg
Then 3-4 days later 15µg
Until he found the threshold dosage. At which point he would invite others to try it so he could see how it effected different people. Then they would compare the effects to other things they had taken and determine the Structure Activity Relationship (SAR).
The Shulgin Scale
+, ++, +++, ++++ or +1, +2, +3, +4
+1= Tipsy
+2= High/Drunk, etc. But you could still go to the store
+3= If you went in public, people would say "I think that person is on something"
+4= Seeing God
And now people say "Threshold/Weak", "Medium", "Strong" in terms of that moments mental effects or the effects of a dosage, but Shulgin had things like "Museum Doses" where he would take like 10mg of 2C-I so that the Museum would come alive.
Sasha mentions the Magical Half Dozen, which is 2C-I, etc
He mentions the 10 Classic Ladies, which is Florence, Ganesha, etc.
But he also mentions that it may be possible to create Molecules using your own liver by turning different enzymes on and off. Then on one of the recipes he gives you a list of all the Phenethylmaines that can be found in your Kitchen's Spice Cabinet, and then throughout PIHKAL he gives you the recipes for each of those Phenethylamines. He says that they just need to be Aminized, either in your liver or in a lab, and they will become active. This theory has been proven by what is now called Oilahuasca. If you mix different things together you can get different effects from different Molecules, it is based on the idea of Pro-Drugs and CYP450 Enzymes (Similar to MAOs which are effected by MAOIs). Some people have reported Psychedelic effects from Mixing just Coffee, Almond, Cinnamon, Vanilla and Nutmeg.

This is similar to how Saffrole, from Sassafras, can be used to make MDA or Ecstasy.
From the person who Discovered Oilahuasca

The benzene ring has 6 positions. In the graphical layout given below the following color guide is used:
position 1 = black (the important allyl side chain, required for conversion to an alkaloid)
position 2 = brown (a methoxy group here seems to cause LSD-like mental effects)
position 3 = red
position 4 = green (must be a methoxy or methylenedioxy group for psychedelic activity)
position 5 = blue (a methoxy or methylenedioxy group here seems to enhance visuals)
position 6 = purple (a methoxy group here probably adds speedy effects)
Position 1 has the allyl side chain hanging off of it. It's the same for all allylbenzenes. This is the part of the allylbenzene that reacts in the body to form a dimethylamine, piperidine, or pyrrolidine alkaloid, if digested properly. The details of this are discussed elsewhere.
In order to have psychedelic activity, position 4 must be a methoxy group. It can be tied to another methoxy group on position 5, as it is with myristicin and others. Two methoxy groups tied together are called a methylenedioxy group.
Position 4 cannot be a hydroxy group as it is in eugenol, hydroxychavicol, and chavicol. This can only lead to stimulant effects, not psychedelic effects.
At the bottom of the chart you can see eugenol, hydroxychavicol, and chavicol. These posses no psychedelic activity, even when properly metabolized. These are the only allylbenzenes in the chart that have a hydroxy group on the 4 position.
Above that we have methyl eugenol, chavibitol, and methyl chavicol. Methyl chavicol and methyl eugenol have psychedelic activity when properly metabolized. The effects of chavibitol are unknown, but are probably like a cross between methyl eugenol and methyl chavicol. The 5 position being a methoxy group seems to improve visual effects.
Above that we have croweacin, apiole, and safrole. Apiole and safrole are psychedelic when metabolized properly. Croweacin is a positional isomer of myristicin. It's activity is not known. It's very likely similar to myristicin, but probably more speedy like apiole. The 6 position being a methoxy group seems to add amphetamine style speedy effects.
Above that we have the ever so popular myristicin, and then dillapiole and the rare sarisan. Both myristicin and dillapiole are psychotic when properly metabolized. The activity of sarisan is unknown. It is a positional isomer of myristicin. It has a methoxy group on the 2 position instead of the 3 position. This probably gives it LSD-like mental effects which are attributed to dillapiole and gamma-asarone rather than myristicin.
Above that we have elemicin, 2,3,4,5-tetramethoxy-allylbenzene, and gamma-asarone. Elemicin is the only one of these that's known to have psychedelic activity when properly metabolized. When properly metabolized, it's effects are similar to myristicin, but more like mescaline. It has the same positional substitutions as myristicin, only the 4 and 5 positions are not tied together. Gamma-asarone is a positional isomer of elemicin and is probably the main active psychedelic compound in calamus oil from Nepal. The methoxy group on the 2 position is probably the reason calamus oil from Nepal has LSD-like mental effects shared by dillapiole and absent from most of the other allylbenzenes. I have no idea if 2,3,4,5-tetramethoxy-allylbenzene is active or not.
For those of you that are unaware, MDMA is primarily manufactured from the safrole found in sassafras essential oil.
Many bioassays of sassafras and even pure safrole show little activity. Usually a slight sedative effect, and very mild MDMA-like effect might be felt if you’re lucky. Although a few people report almost full blown MDMA-like effects from sassafras as is, most people get no effects. This points in the direction of human metabolism altering the effects of safrole.
AFOAF once tried sassafras with a CYP1A2 inhibitor: German chamomile. This experiment produced stimulant effects unlike the effects had from just sassafras alone. There was mild euphoria, and some possible mild visuals effects.
Safrole is primarily inactivated by conversion into 1-hydroxysafrole by CYP2A6. German chamomile primarily inhibits CYP1A2, so AFOAF was using the wrong inhibitor, but it was somewhat effective.
Cinnamon bark oil is a potent CYP2A6 inhibitor. This should theoretically prevent CYP2A6 from turning safrole into 1-hydroxysafrole.
My theory is that about 2-5 grams of cinnamon, or 5-10 drops of cinnamon essential oil will contain enough cinnamaldehyde to inhibit CYP2A6 and allow MDMA-like effects to be experienced from sassafras or sassafras oil.
This is only a theory. As of yet AFOAF has not tried this. He will try this very soon. Incidentally, it is the 1-hydroxysafrole which is primarily formed by CYP2A6 which is considered weakly carcinogenic and not safrole itself. So by using cinnamon oil, the weak potential carcinogenicity of safrole should be greatly reduced.
For his test he plans on using German chamomile oil at 3 drops along with the cinnamon bark oil at 6 drops. He will use sassafras bark, freshly ground, extracted into milk with lecithin, just like how you make kava. This is the best way to extract the safrole without using a solvent. He will take the sassafras milk and the oils at the same time. Hopefully this produces an MDMA-like effect. We will see.
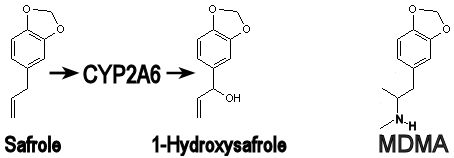
Here's a picture of what the CYP2A6 enzyme in humans does to safrole. Once converted to 1-hydroxysafrole by CYP2A6, it becomes much more polar (the XLogP drops from 3 way down to 1.9!), and so it's far more difficult for it to enter the brain, meaning it should be far less psychedelic (assuming that safrole is actually psychedelic before this conversion). For maximum entry into the brain, CYP2A6 should be inhibited to prevent this conversion. It’s possible that CYP1A2 might be able to perform this very same conversion (it does so with methyl chavicol and elemicin), so it too should be inhibited.
On the right you can see MDMA for comparison purposes. See how 1-hydroxysafrole is less like MDMA than safrole is? The XLogP3 of safrole is 3, MDMA is 2.2, and 1-hydroxysafrole is 1.9, being the most polar of the three. Safrole should more easily cross the blood brain barrier than MDMA, but once attacked by CYP2A6 enzymes, safrole turns into 1-hydroxysafrole, and that should help prevent it from entering the brain.

Later...
Well it was tried a few hours ago.
AFOAF used 5 grams of sassafras bark, 1 gram of lecithin, ground to powder, then mixed with 1 cup of steaming hot milk and 2 ml of vegetable oil. This was left to sit for 2 hours and was then filtered. It was super hard to filter. Decanting would be a better idea.
He then mixed 6 drops of cinnamon bark oil and 3 drops of German chamomile oil into it. The German chamomile would not mix into it and just floated to the top. He mixed it as good as he could and drank it down.
Its been about 3 hours and he feels NICE. There's very obvious euphoria. Sense of touch is enhanced. He feels good. There's no sedation felt at all. It’s mildly psychedelic.
This seemed to work. But he doesn’t know what MDMA really feels like so he can’t compare it to MDMA. It does feel like a phenethylamine though. It’s very different from taking sassafras on its own.
This is a light dose. I think 10-20 grams of bark would be much better.
I would love to hear what others more familiar with MDMA think about this combination. It seems to have worked to produce an MDMA-like effect, but until others more familiar with MDMA test this out, we should take that statement as simply a guess.
Procedure
Procedure, in plain English:
Pepper would be made into a tea. Solids filtered out.
Then you would get some Anise Oil, B9 or Valerian Root (of Chinese Origin)
So that is your Pepperidine, and you activators. Now you need your Enzyme Inhibitors. You can add L-Lysine, but it is not necessary.
Vanilla and Cinnamon work, pick one or both. You also need the Aldehyde structure from one of these.
Next. German Chamomile, Cayenne Pepper Capsules or Tangerine Skin extract/capsules
Then
Almond extract, Anise Oil (if you already had it), Cinnamon, Lemon peel oil, Lime peel oil, or a cigarette or nicotine gum if you can't find anything else.
Then
CBD, Echinacea Purea, Pomegranate, Pummelo, or Calamus Oil.
Then
Clove oil, Catechin, Dill seed Oil or Goldenseal.
Then Kudzu or Glycerin or Caffeine
Not all of these things are neccisarry, but if you do 1 thing in each list, you should get very strong effects from whatever you take.
The best thing to take is Sweet Basil Extract, in it's pure form, it is known as Methyl Chavicol.
Take all that other stuff like 30 minutes to an hour before the Basil Extract, and redose the B9, Anise or Valerian root to keep the effects going without taking more.
Science
Graviola- 5-HT1a Agonist
Black Cohosh- 5-HT1A, 5-HT1D & 5-HT7 Binding
C. Foetida L.- 5-HT1A Agonist
Yokukansan- 5-HT1A Agonist
DMT hits all of these, and can be found in tons of plants.
Black Cohosh- 5-HT1D
maybe Rhodiola rosea, Albizia lebbeck & Albizia julibrissin.
5-HT1 Receptor Agonists:
Turmeric, Ginger, Ginko Bilboa, Lemon Essential Oil, Rauwolfia, Valerian, Yohimbe
Elmicin & Myristicin (in Nutmeg)- 5-HT2A Agonist
Estragole (in Sweet Basil)- 5-HT2A Agonist
Safrole (in Sassafras)- 5-HT2A Agonist
Cinnamon Bark- CYP2A6 & CYP2E1 Inhibitor (It will deplete your liver's Glutithione) Taken 1 Hour before Allybenzene,
Clove Leaf- CYP2C9, CYP3A4, CYP1A1 & CYP1B1 Inhibitor
German Chamomile- CYP1A2 Inhibitor (Caffeine may also do this)
GoldenSseal & Echinacea purpurea very effectively do the same thing.
Black seed oil, 50% EGCG, Valerian root oil, Pomegranate, Vitamin B9, 40% Ellagic extract, Rooibos 20% Gallic acid extract, Rutin, B3 & Kudzu
AllylBenzenes
Anethole, Apiol, Asarone, Carpacin, Chavibetol, Chavicol, Dillapiole, Eugenol, Isoeugenol, Isosafrole, Methyl Eugenol, Methyl Isoeugenol,
since Cinnamon is a Phenylpropanoid, and Phenylpropanoids are made from Phenelalamine, and people who took Phenelalamine claim to get better results. I decided to post a list of Phenylpropanoids also.
Caffeyl Alcohol, Cinnamaldehyde, Cinnamyl alcohol, alpha-Cyno-4-hydroxycinnamic acid, Ethyl Cinnamate, Lignin, 2,4-Methlenedioxypropiophenone, Neoflavonoids, Nordihydroguaiaretic acid, Phenylpropanoic acid, Phloretic acid, Rhododendrin & Suberin.
Star Anise Extract or B9 for CYP2C9 Induction
NMDA Receptor Plants:
Uncaria Rhynchophyllia
Psychotria Colorata
Huperzia Serrata
Most important things:
CYP2C9 Induction
Alcohol Dehydrogenase Induction
Aldehyde Dehydrogenase Inhibition
Piperidine and or Dimethylamine Supplementation
Methyl from foods
Exercise or compounds that produce effects like exercise
Less important, but still factors:
SSAO Inhibition (Caffeine, Phenethylamine, Phenelalamine, Tryptamine)
MAO-A Induction
MAO-B Induction
NDMA Antagonism
Prolactin Inhibition
Hungarian Parsley Seed is a better source of Myristicin than Nutmeg. The effects of it when activated properly are said to be like Mescaline and MDMA together. The P450 Enzymes CYP1A2 & CYP3A4 are what break this down and need to be inhibited. CYP2D6 could also play a big role.
Elmicin is something you either need Chromotography type knowledge to get, or you have to buy it in small quantities. When activated properly it is like Mescaline, when activated wrong it is like Melatonin (sleepy). CYP1A1, CYP1B1, CYP1A2, CYP2A6, CYP2C9, CYP2A6, CYP2C9 & CYP2E1 are what are needed to be inhibited to activate this. CYP2D6 could also play an important role.
Safrole is like MDMA when activated properly and like Melatonin when not. CYP2A6, CYP2C9, and CYP2E1 are most important for this. CYP2D6 could also be important.
Methyl Chavicol when activated properly is like a light speedy LSD, when activated wrong it is said to be almost like Marijuana. CYP1A2 and CYP2A6 inhibit it, and CYP2D6 could also be important.
If the CYP2D6 Enzyme is inhibited with all the others, these are possibly visually hallucinogenic Oilahuascas. And the Methyl Chavicol doesn't build a tolerance (the others do) it actually gets stronger for you every time you use it, or you can use less.
Several allylbenzenes have been proven to form up to 3 alkaloid metabolites after ingestion by several animals.[2][3] They do not form amphetamines in vivo as has been speculated in the past. The alkaloids detected in animal urine are tertiary aminopropiophenones of 3 possible subtypes: dimethylamines, piperidines, and pyrrolidines.[1][2][3][4]
The allylbenzene elemicin has been proven to form all 3 different alkaloid metabolites after ingestion in animals by analyzing urine using gas-liquid chromatography and chemical ionization mass spectrometry.[1]
Safrole is also proven to form all three alkaloid metabolites after ingestion.[2]
Myristicin appears to only form piperidines and pyrrolidines. Dimethylamines of myristicin have not been detected.[3]
Allylbenzene, from which all allylbenzenes are derived, forms piperidine and dimethylamine alkaloids.[4]
Propenylbenzene and its derivatives (asarone, anethole, etc.) do not form alkaloid metabolites.[4]
Here is how it works
CYP Enzymes (Drug Metabolism, etc)
Induction and Inhibition (Anti-Oxidants, etc)
All inhibitors of oxidative 17bHSD2 will prevent activation of allylbenzenes. This enzyme must be induced, not inhibited. It's the single most important enzyme to induce. If oxidative 17bHSD2 is not functioning, allylbenzenes cannot produce psychedelic activity. Naringenin also potently inhibits 17bHSD2. Grapefruit contains large amounts of naringenin, and also prevents the psychedelic action of allylbenzenes if taken before allylbenzenes. Inhibition lasts approximately 4-8 hours.
Oilahuasca Diet
Here's a list of all known 17bHSD2 inhibitors that should be avoided 4-8 hours prior to using allylbenzenes:
•Quercetin and all food or supplements containing large amounts of it.
•Apples (0.0263% quercetin)[6]
•Cabbage (0.01% quercetin)[6]
•Cranberry (0.025% quercetin)[6]
•Evening-Primrose (20% quercetin)[6]
•Galangin and all food or supplements containing large amounts of it.
•Garlic (0.02% quercetin)[6]
•Grapefruit (contains naringenin, kaempferol, galangin, and quercetin)
•Himalayan Mayapple (1.2% quercetin)[6]
•Kaempferide and all food or supplements containing large amounts of it.
•kaempferol and all foods that contain large amounts it.
•Mayapple (5% quercetin)[6]
•Naringenin and all food or supplements containing large amounts of it.
•Neem (0.1% Quercetin)[6]
•Oats (0.031% Quercetin)[6]
•Onions (4.81% quercetin).[6]
•Orange (4.58% naringenin)[6]
•Tea (10-25 mg/L quercetin, 6.3-17 mg/L kaempferol [7]).[6]
•Tomato (contains kaempferol, naringenin)
•Yuzu (contains naringenin)
Tasting
Sasha used to invent brand new Molecules that had never been recorded before, and then he would taste them to discover their effects.
Here is how tasting works.
He would invent a new Molecule, similar in structure to an existing molecule. And he would use different tests to see what the Molecule looked like, for example he had a machine that could tell him a few things, and if you put bromide on a molecule and it turns to blood colored or white you can tell it has different things attached to it.
Then, he would take this new Molecule, measure out 1µg and eat it.
Then over 3-4 days he would record the effects, if any, using a scale of +, ++, +++ or ++++
And the 3rd or 4th day he would take 5µg
Then 3-4 days later 10µg
Then 3-4 days later 15µg
Until he found the threshold dosage. At which point he would invite others to try it so he could see how it effected different people. Then they would compare the effects to other things they had taken and determine the Structure Activity Relationship (SAR).
The Shulgin Scale
+, ++, +++, ++++ or +1, +2, +3, +4
+1= Tipsy
+2= High/Drunk, etc. But you could still go to the store
+3= If you went in public, people would say "I think that person is on something"
+4= Seeing God
And now people say "Threshold/Weak", "Medium", "Strong" in terms of that moments mental effects or the effects of a dosage, but Shulgin had things like "Museum Doses" where he would take like 10mg of 2C-I so that the Museum would come alive.
Last edited:



