-
Neuroscience & Pharmacology Discussion Welcome Guest
Posting Rules Bluelight Rules Recent Journal Articles Chemistry Mega-Thread FREE Chemistry Databases! Self-Education Guide -
N&PD Moderators: Skorpio | someguyontheinternet
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Easest way of modifying Methamphetamine, to something else and keep most of effects?
- Thread starter InsaneSpeedball
- Start date
InsaneSpeedball
Greenlighter
- Joined
- Feb 7, 2007
- Messages
- 8
Jamshyd said:I'm telling you, its a known fact that these things are VERY TOXIC.
In fact, they are usually blamed for much of Meth's toxicity because 4-Iodo-meth is found as an impurity in street meth.
PLEASE don't do this!
EDIT: I WANT TO STRESS THE TOXICITY. These compounds are KNOWN to cause long-term serotonin depletion.
Ooops!
That was a spooky suprise for me.
And leads to other questions, what about DOB, DOI,DOC and 2CB,2CI, 2CC, are they neorotoxic as well? Or is just methamphetamine with halos attached to them.
InsaneSpeedball said:Ooops!
That was a spooky suprise for me.
And leads to other questions, what about DOB, DOI,DOC and 2CB,2CI, 2CC, are they neurotoxic as well? Or is just methamphetamine with halos attached to them.
it doesn't appear that DOB DOI or DOC are neurotoxic, at least from the limited data available. they are very different pharmacologically from the p halo amphetamines, none of these agents act directly as serotonin or dopamine releasers and none interact with the various dopamine and serotonin transporter proteins.
it is presumed that the 4-halomethamphetamines (not F) are neurotoxic because they are metabolised to 4-halo amphetamines which are known neurotoxins. there are no studies that I am a aware of that have specifically examined the halo meth amphetamines for neurotoxicity, however 30% of the halometh eaten is going to end up being the neurotoxic amphetamine. The mechanism of the neurotoxicity of say 4 chloroamphetamine PCA is not fully understood, it is a serotonin releaser which causes a short and long term effects, short term it increases serotonin levels, then there is depletion presumably once the serotonin has mostly been released then days to months later it kills serotinergic neurons.
In all there are quite a few potent neurotoxins related to amphetamine, dopamine and serotonin.
giantsquid
Bluelighter
- Joined
- Nov 10, 2005
- Messages
- 352
InsaneSpeedball said:Ok, that means that every substance that is very simular to an illegal one, will be by auto, illegal to? How long does is stretch? And what is the exakt defination, rules for calling something an analog, how simular must it be?
Ok, were I live, I don't think we have that law...yet. Some new substances that comes up, either get schedule narcotic, or some is just put under a section called, something like "Unhealthy Goods", which means they are not allowed to be selled. But not illegal to possess. And things takes time here.
DOI for example is still legal so far. Which is a pretty heavy drug.
The USA laws are so vague that if you have a drug that does similar things to an illegal drug then it could be classed as an analog even if it is not chemically similar
this is from erowid
The Federal Analogue Act defines an analog as a substance which is 'substantially similar' to a scheduled substance and has either an effect 'similar to or greater than' a controlled substance or is thought to have such an effect. The law fails to define what 'substantially similar' means, nor does it try to clarify what would constitute a 'similar or greater' effect.
The explicit intention of Congress when they created the law was to be able to allow the DEA to arrest and prosecute underground chemists who make minor changes to an existing illegal chemical, resulting in a new chemical which can be sold as a recreational drug but which is not specifically listed as illegal. Because the law is written so broadly and vaguely, however, it is considered by experts in the field of psychoactive chemistry to be one of the most oppressive laws ever written, making the very creation of a new, unknown and untested chemical, illegal.
vecktor said:The mechanism of the neurotoxicity of say 4 chloroamphetamine PCA is not fully understood, it is a serotonin releaser which causes a short and long term effects, short term it increases serotonin levels, then there is depletion presumably once the serotonin has mostly been released
then days to months later it kills serotinergic neurons.
Do you mean 4-chloroamphetamine with "it" ? I'm interested in this mechanism are there any refs that point to this mechanism?
Vaque said:Do you mean 4-chloroamphetamine with "it" ? I'm interested in this mechanism are there any refs that point to this mechanism?
yes, and I have not seen a paper which discusses the mechanism of PCA toxicity, which apparently looks different to fenfluramine and MDMA/MDA
nuke
Bluelighter
- Joined
- Nov 7, 2004
- Messages
- 4,191
4-Cl-AMP causing heavy oxidative damage to SERT and serotonin axons, effectively destroying them. The brain afterwards has no means of regenerating them, so you lose them forever and ever. I think it's not very different from MDMA/MDA/meth, I'd bet there'd be evidence of ortho-oxidation of the drug in rat brains if you excised them before the drug was excreted. But, maybe, it's something else.
Vaque said:Can you explain me then perhaps how it is possible that 4-chloroamphetamine would destroy serotinergic neurons months later, does this compound reside in the brain for that long ?
it doesn't have to, it merely has to do enough damage so that programmed cell death is initaited at a future point. a few toxins are capable of destroying cells long after the toxin itself has gone, they merely cause enough damage and the cellular machinery does the rest either increasing the damage or recognising the damage and triggering apotopsis, I sppose some of them could have application as undetectable poisons.
nuke said:4-Cl-AMP causing heavy oxidative damage to SERT and serotonin axons, effectively destroying them. The brain afterwards has no means of regenerating them, so you lose them forever and ever. I think it's not very different from MDMA/MDA/meth, I'd bet there'd be evidence of ortho-oxidation of the drug in rat brains if you excised them before the drug was excreted. But, maybe, it's something else.
apparently the damage caused by PCA is distinguishable from that caused by MDMA on dissection, I am not 100% certain that this claim which was made during the dexfenfluramine controversy has been validated since.
Helios.
Ex-Bluelighter
In my country we still have Freedom of Speech, and I like to talk and I know a lot and that's about it and I don't care.
PCA was a common FDA approved antidepressant used in the 1970's with no noticeable, much less irreversible, neurotoxic side effects until scientists found morphologic post mortem brain changes. The exact same changes can today be found from any SSRI.
Compare the LD50's of DOB, MDA, MDMA, and methamphetamine and tell me which is most to least neurotoxic. Of course, this assignment is no carte blanche / tableau rossa to go consume a lot of 4-Br-METH; however, if you do keep in mind that
4-Cl-METH is less toxic. (F may be electronegative, but it is hardly bigger than a lone hydrogen atom.)
Cuminaldehyde is a better route but.
Isopropyl alcohol + HCl or better yet SOCl2 --> 2-chloropropane
methamphetamine + 2-chloropropane + AlCl3 (Freidels Craft conditions) -->
Frankinmeth or, if you'd rather, Myrhhmeth.
The latter would likely require chromatographic separation of components, the former would not.
Hope this helps!
However if you do it the hard way, you will also have to first protect the meth's secondary N by making it into an amide (with/from perhaps trifluoroacetic acid) I guess, then do the Freidel Crafts, then deprotect ie destroy the protecting goup. Also, more than one isopropyl group may add onto some of the benzene rings.
This is just organic chemistry talk.
PCA was a common FDA approved antidepressant used in the 1970's with no noticeable, much less irreversible, neurotoxic side effects until scientists found morphologic post mortem brain changes. The exact same changes can today be found from any SSRI.
Compare the LD50's of DOB, MDA, MDMA, and methamphetamine and tell me which is most to least neurotoxic. Of course, this assignment is no carte blanche / tableau rossa to go consume a lot of 4-Br-METH; however, if you do keep in mind that
4-Cl-METH is less toxic. (F may be electronegative, but it is hardly bigger than a lone hydrogen atom.)
Cuminaldehyde is a better route but.
Isopropyl alcohol + HCl or better yet SOCl2 --> 2-chloropropane
methamphetamine + 2-chloropropane + AlCl3 (Freidels Craft conditions) -->
Frankinmeth or, if you'd rather, Myrhhmeth.
The latter would likely require chromatographic separation of components, the former would not.
Hope this helps!
However if you do it the hard way, you will also have to first protect the meth's secondary N by making it into an amide (with/from perhaps trifluoroacetic acid) I guess, then do the Freidel Crafts, then deprotect ie destroy the protecting goup. Also, more than one isopropyl group may add onto some of the benzene rings.
This is just organic chemistry talk.
Last edited:
Helios. said:In my country we still have Freedom of Speech, and I like to talk and I know a lot and that's about it and I don't care.
PCA was a common FDA approved antidepressant used in the 1970's with no noticeable, much less irreversible, neurotoxic side effects until scientists found morphologic post mortem brain changes. The exact same changes can today be found from any SSRI.
Compare the LD50's of DOB, MDA, MDMA, and methamphetamine and tell me which is most to least neurotoxic. Of course, this assignment is no carte blanche / tableau rossa to go consume a lot of 4-Br-METH; however, if you do keep in mind that
4-Cl-METH is less toxic. (F may be electronegative, but it is hardly bigger than a lone hydrogen atom.)
!
am I missing something what is the product of the above reaction scheme(s)??
Last edited by a moderator:
Helios.
Ex-Bluelighter
para-isopropylmethamphetamine hcl or "PIMA."
fastandbulbous
Bluelight Crew
vecktor said:it doesn't have to, it merely has to do enough damage so that programmed cell death is initaited at a future point. a few toxins are capable of destroying cells long after the toxin itself has gone, they merely cause enough damage and the cellular machinery does the rest either increasing the damage or recognising the damage and triggering apotopsis, I sppose some of them could have application as undetectable poisons.
Pitching for a job with the KGB/CIA vecktor?
 They need some help judging by their previous track records!
They need some help judging by their previous track records!Helios.
Ex-Bluelighter
Who buys / needs / intends perhaps to use 10's of millions of grams of
2,5-dimethoxyamphetamine hcl per year? I don't care to know. See the P Book for ref. if you don't believe me but you want to.
2,5-dimethoxyamphetamine hcl per year? I don't care to know. See the P Book for ref. if you don't believe me but you want to.
Helios. said:Who buys / needs / intends perhaps to use 10's of millions of grams of
2,5-dimethoxyamphetamine hcl per year? I don't care to know. See the P Book for ref. if you don't believe me but you want to.
eastman kodak as was
Reminisant B
Greenlighter
- Joined
- Nov 3, 2005
- Messages
- 401
hussness
Bluelighter
vecktor said:eastman kodak as was
You don't seriously expect me to believe they were using it to make cameras!
Jamshyd
Bluelight Crew
^ No, but they do use Hydroquinone, a precursonr of DMA-2 and several 2Cs (I believe).
fastandbulbous
Bluelight Crew
Reminisant B said:I read this somewhere else but thought it might be a useful idea in this thread.
Looks possibly toxic (MPTP comes to mind)
Still hadn't thought of it until I read it elsewhere.
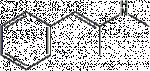
Unsaturated Meth
Wont work as it'll rearrange into the N-methyl imine (which will hydrolyse to benzyl methyl ketone/phenylacetone and methylamine) a la keto-enol tautomerism