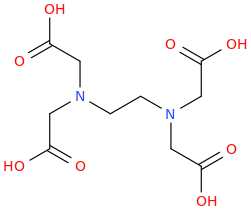
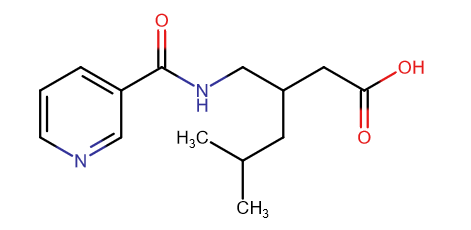
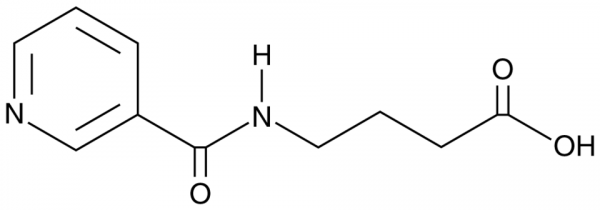
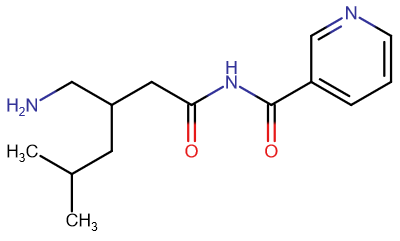
polymath
Bluelight Crew
- Joined
- Nov 4, 2010
- Messages
- 1,884
Some species really do have more effective kidney function.I wonder if that rate is faster in dogs? One would imagine that if dozens of them were doped and the effects lasted for weeks, it would be quite evident.