Dextro .45
Bluelighter
- Joined
- Apr 15, 2017
- Messages
- 789
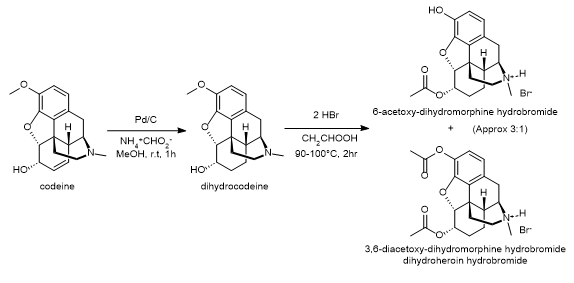
Diacetyldihydromorphine (also known as Paralaudin, dihydroheroin, acetylmorphinol) is a potent opiate derivative developed in Germany in 1928 which is rarely used in some countries for the treatment of severe pain such as that caused by terminal cancer, as another form of diacetylmorphine (also commonly known as Heroin). Diacetyldihydromorphine is fast-acting and longer-lasting than diamorphine, with a duration of action of around 4-7 hours.[1]
Diacetyldihydromorphin
Diacetyldihydromorphin
This would be the ULTIMATE Heroin opioid in pharmaceutical glass ampoules for IV/IM/SC injection Germany has the best drugs on earth…..it’s Faster acting & Longer lasting than Diacetylmorphine (Heroin) Some professional pharmaceutical laboratory could synthesize this molecule like one would bake a cake 100% purity in a multi use glass 60ml bottle 100kg (220 lbs) of pure API compounding HCL powder in a large secure drum/container in a dark cold cellar I would LOVE to inject pharmaceutical grade powder of this with Pharma Cocaine HCL Why hasn’t this amazing Diamorphine improved analog not been illicitly synthesized and available for bulk purchase | |
|---|---|