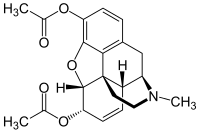
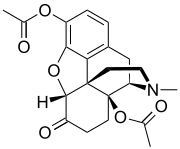
Well, the wiki page on the 3,14 di--- also mentions a "3,6,14-triacetyloxymorphone" and my personal favorite " 3,6,8,14-tetraacetyloxymorphone"....so because of all the really awesome bubble hash I just smoked, I'm going to believe everything on this wiki page ( dispite it having one source from a United Nations Office on Drugs and Crime bulletin from the early fifties) and in this fantasy land I'm going to ask what the extra 14 (or 8 AND 14) would/could/might do to the 3,6 diacetyl I was dreaming of in the first place- my theory is the 14 causes enough reverse tolerance that by week 2 of a real good run your habit is netting you like 18$ profit a day (is delusional reverse price discussion allowed?) and the and the 8 works like an airbag that only kicks in if your gonna stop breathing and releases just enough of an antagonist to bring you back to buzzed. (again, not a chemist)
edit seriously like a second later:
Although.... a search for the Triacetyl does bring up a bunch of instances in patents that contain way too much synth to post (i think) where they either found or specifically noted that they didnt find "3,6,14-triacetyloxymorphone"