Hammilton
Bluelighter
- Joined
- Sep 2, 2008
- Messages
- 3,435
I've meant to start this thread for a long time, because there have been a lot of threads on various old depressants, but they're all either in singles or doubles- and I don't think we've remotely run out of old depressants to discuss.
Of course, though, limiting yourself to discussion of the old drug isn't good, but discussions of LOGICAL variants of the old drugs should be in here too.
To start the discussion, I want to talk about a drug called propanidid, which was taken off the market because of allergic reactions, though today it is believe to have been due to a component of the product, not propanidid itself.
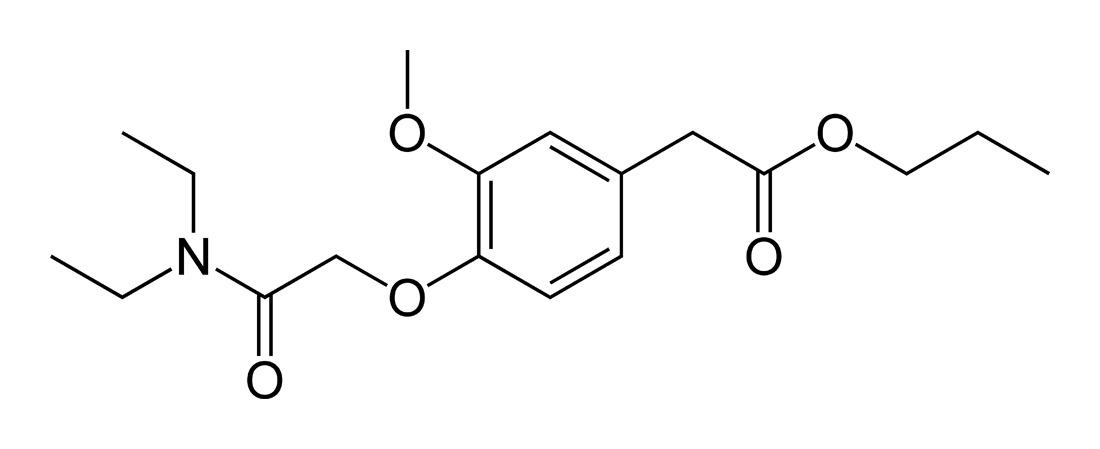
The structure reminds me dimethocaine, actually. Too short acting to have any da reuptake inhibiting effects, though. A strange symmetry, too. It's interesting that it has analgesic effects too. Most GABAergic depressants don't reduce pain, and many actually produce hyperalgesia, which makes this so interesting.
I know it has effects on the GABA-A receptor, but I don't know where it binds.
This is very interesting, but I don't know what to make of it. I wouldn't and still don't think that opioid receptors are very involved in the sedation produced by GABAergics, but apparently, at least in the transfer from consciousness to unconsciousness, for some, they are. I don't understand why drugs effecting the same receptor would have different downstream effects. I mean, they both cause the chloride channel to stay open longer or open more frequently, and there's no obvious split between those that cause it to open more often and those that cause it to stay open longer.
Any explanation?
I think the biggest goal with propanidid would be to produce a longer acting analogue. I believe it has a plasma halflife of .2 hours, or 12 minutes. That seems in line with it's ultra-short effect. Great for anaesthesia where you want the person to come out quickly, not so great for abuse. Depressant crack sounds interesting, but it's a bad idea.
I think at least a 30 minute half life would be required, but 60 minutes would probably be best. At least, imho, anyway. I like a depressant that hits rapidly and is over in 1.5 or 2 hours. A 60 minute half life should be good for that. It's hard to predict though.
Of course, though, limiting yourself to discussion of the old drug isn't good, but discussions of LOGICAL variants of the old drugs should be in here too.
To start the discussion, I want to talk about a drug called propanidid, which was taken off the market because of allergic reactions, though today it is believe to have been due to a component of the product, not propanidid itself.

The structure reminds me dimethocaine, actually. Too short acting to have any da reuptake inhibiting effects, though. A strange symmetry, too. It's interesting that it has analgesic effects too. Most GABAergic depressants don't reduce pain, and many actually produce hyperalgesia, which makes this so interesting.
I know it has effects on the GABA-A receptor, but I don't know where it binds.
The Analgesic Action and Neuronal Mechanism of Propanidid Tatsushi Fujita, MD,PhD, Hideaki Ishikura, MD,PhD Yasuharu Kitani, MD British Journal of Anaesthesia, 1972, Vol. 44, No. 8 809-816 © 1972 The Board of Management and Trustees of the British Journal of Anaesthesia
Subanaesthetic doses of propanidid, thiopentone and ketamine were given to volunteers at 30 min intervals. Change in the pain threshold values were observed by means of the earlobe algesimeter. The pain threshold value sharply rose 30 sec after propanidid 1 mg/kg but returned to control values in 2 min. With ketamioe 0.1 mg/kg, the pain threshold rose in 2 min and the rise was maintained over 5 min. Thiopentone 0.5 mg/kg showed no significant change. Further, in order to clarify this analgesic effect, experiments were performed on both intact and cerveau isolé rabbits to observe the effects of propanidid on the sensory pathways of the olfactory and visual systems. Propanidid had less inhibitory effect on the monosynaptic than on the polysynaptic reflex thus differing from the effects of barbiturates which inhibit both mono- and polysynaptic reflexes. Study of the recruiting response on the cortex after stimulation of the centre-median nucleus indicated that propanidid transiently inhibits the thala-mocortical pathway. The neuronal mechanisms are evidence of the existence of an analgesic property of propanidid.
This is very interesting, but I don't know what to make of it. I wouldn't and still don't think that opioid receptors are very involved in the sedation produced by GABAergics, but apparently, at least in the transfer from consciousness to unconsciousness, for some, they are. I don't understand why drugs effecting the same receptor would have different downstream effects. I mean, they both cause the chloride channel to stay open longer or open more frequently, and there's no obvious split between those that cause it to open more often and those that cause it to stay open longer.
Any explanation?
Effect of Naloxone on the Loss of Consciousness Induced by IV Anaesthetic Agents in Man. Stella, L. Crescenti, A. Torri, G. British Journal of Anaesthesia, 1984, Vol. 56, No. 4 369-373 © 1984
Correspondence
The effect of a specific opioid antagonist, naloxone, was studied in two comparable groups of patients who received i.v. the dose of an anaesthetic agent required to produce loss of consciousness in 50% of subjects. The first group received naloxone 0.006 mg kg–1 5 min before induction of anaesthesia; the second group received a similar volume of saline solution. Thiopentone, Althesin, diazepam, ketamine and propanidid were studied. The differences in percentage of unconscious patients between the naloxone-treated group and the control group were statistically significant for diazepam, ketamine and propanidid. Naloxone did not modify the induction of anaesthesia with thiopentone or Althesin
I think the biggest goal with propanidid would be to produce a longer acting analogue. I believe it has a plasma halflife of .2 hours, or 12 minutes. That seems in line with it's ultra-short effect. Great for anaesthesia where you want the person to come out quickly, not so great for abuse. Depressant crack sounds interesting, but it's a bad idea.
I think at least a 30 minute half life would be required, but 60 minutes would probably be best. At least, imho, anyway. I like a depressant that hits rapidly and is over in 1.5 or 2 hours. A 60 minute half life should be good for that. It's hard to predict though.