LikeADaVinci
Bluelighter
- Joined
- Dec 31, 2023
- Messages
- 25
Hi, I’m new here, but I’ve been studying receptor theory, structure activity relationships, receptor theory, molecular docking theory & etc for almost twenty years.
I wanted to propose a few new amphetamine analogues for you all to consider (and possibly synthesize and try out if you have the time & ability)
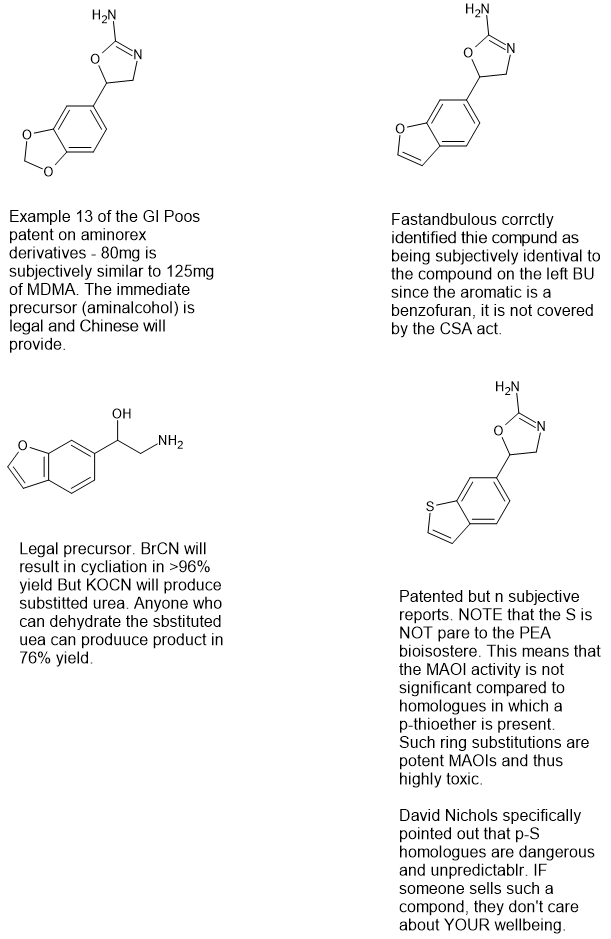
They are both derived from aminorex, I call them amphetaDiAmines since there are two amines:
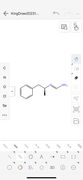
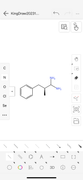
Since aminorex contains two amines, I have deconstructed the ring structure to lay out the amines linearly
Can anyone put these chemicals into software to guess the monoaminergic activity for binding affinities of NA, DA, & 5-HT?
Please let me know what you think, bluelighters!
I wanted to propose a few new amphetamine analogues for you all to consider (and possibly synthesize and try out if you have the time & ability)
They are both derived from aminorex, I call them amphetaDiAmines since there are two amines:


Since aminorex contains two amines, I have deconstructed the ring structure to lay out the amines linearly
Can anyone put these chemicals into software to guess the monoaminergic activity for binding affinities of NA, DA, & 5-HT?
Please let me know what you think, bluelighters!
Last edited: