Using the idea that to show any great degree of activity the compound(s) need to roughly fit the structure of D-LSD/serotonin in terms of bond length, stereochemistry and lone pair electron orientation for formation of hydrogen bonds.
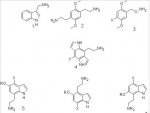
Out of the structures shown, 2 is effectively a 2C-x compound, it's chemical name shows that more clearly when written as a 2C-x type compound: 4-(2-aminoethyl)-2,5-dimethoxyphenethylamine. Any activity will be dependant upon the lipophillic properties of the 2-aminoethyl group (it may turn out to be too hydrophillic to be very active), but who knows - it should have some sort of activity.
4 is a phenethylamine version of a dragonfly, but with nitrogen atoms where the oxygen atoms should be. As the 5-methoxy/furanyl oxygen of phenethylamine hallucinogens effectively fills the role of the indolic nitrogen, the two should be interchangable to a certain degree. Both have at least one lone pair of electrons for forming hydrogen bonds to the receptor protein, so structure 4 should work in theory.
5 & 6 are on the same principal as 4, only they are hemi-dragonflies with the indolic nitrogen replacing the oxygen in the one furanyl ring of said hemi-dragonfly. Because of this, the only useful groups attached to the sidechain nitrogen are likely to be hydrogens - alkyl groups will be like N-alkylating the nitrogen of phenethylamines.
7 is a complete unknown. It has the 2,4,6 type of substitution pattern, but as no studies have been carried out w.r.t. extending a methoxy group to a furanyl/dihydrofuranyl ring, they're anybody's guess (must say though, something tells me no, or not very likely as the lone pairs are just not in optimal position for formation of hydrogen bonds.
The out of position lone pair electrons idea also applies to compound 1. Compound 3 also seems unlikely as the 'stereochemistry' (I know there's no stereochemistry as such - I was just referring to bond length and angles) is wrong as well as the influence of the electronegativity of the oxygen atom on the lone pair orientation of the nitrogen atom (which is needed to form hydrogen bonds to receptor protein).
Although that's just my view from understanding the spatial orientation and positioning of charges/lone pairs of the compounds, the big test is to synthesise them and do receptor affinty trials.
PS I find .doc files crap to deal with, so I've redone the image as a jpeg