C6H6
Bluelighter
- Joined
- Jan 29, 2005
- Messages
- 607
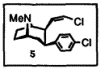
I've been reading up on cocaine analogs a bit lately and came across one intriguing compound which is briefly mentioned in one paper as not only being very potent, but also that it lasts up to 3 days in rodents because all ester functions are eliminated thus esterases can't break it down.
Maybe someone could retrieve the full paper and make it avaliable to us?
Bioorganic & Medicinal Chemistry Letters
Volume 3, Issue 6 , June 1993, Pages 1327-1332
http://tinyurl.com/dnhb6
Maybe someone could retrieve the full paper and make it avaliable to us?
Bioorganic & Medicinal Chemistry Letters
Volume 3, Issue 6 , June 1993, Pages 1327-1332
http://tinyurl.com/dnhb6