Has anyone else had the chance to try chlormezanone? GB 815203
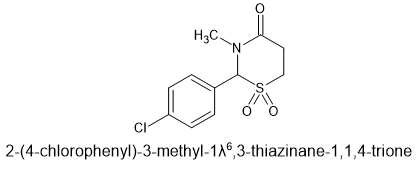
I only got to try a single 400mg tablet but it was quite potent. It reminds me once again that sedative/hypnotics always seem to have an amide and that tertiary amides are the more potent. Chlormezanone binds at the same site as the benzodiazepines and mixed with diazepam (Valium) it was taken as a cocktail called 'Fenavan' or 'Vans' for short. What was interesting was that it didn't seem to matter if it was a 1,4-benzodiazpine or a 1,5-benzodiazpeine (clobazam is the only example that springs to mind.
I do not know who invented this mixture but the sedative effects were far more pronounced although in it's favour, it did not seem to produce the aggression that some benzo cocktails cause. It was most certainly something you took as you sat down to watch a TV film.... so you could wake up as the credits went up the screen.
I did spend a bit of time looking for derivatives but it's low potency meant that it was not a useful target.
It's widely used within China and I do not think their is any legal control....
One Alexander R Surrey (Sterling Drug Company) spent a good decade of his career researching ths class and several
analogues proved to be more active but as usual, as soon as the marketing people found SOMETHING from which
to profit, they marketed it (and 1950s safety trials were not exactly great).
US3093639A
US3155655A
US3244703A
CN104817518A
I freely admit that I have not plowed through every related paper & patent but I am told that several homlogues are much like methaqualone. It's just the luck of the draw. One became a huge hit, the other is a footnote.
BTW I just remembered that the two enantiomers are said to possess quite different properties. The ADME of both are roughly the same but only one is an active anxiolytic. I believe that initially the designers intended to resolve the isomers BUT the only significant advantage was a faster onset and shorter duration - not big advantages for a medicine, possibly an advantage for an RC.

I only got to try a single 400mg tablet but it was quite potent. It reminds me once again that sedative/hypnotics always seem to have an amide and that tertiary amides are the more potent. Chlormezanone binds at the same site as the benzodiazepines and mixed with diazepam (Valium) it was taken as a cocktail called 'Fenavan' or 'Vans' for short. What was interesting was that it didn't seem to matter if it was a 1,4-benzodiazpine or a 1,5-benzodiazpeine (clobazam is the only example that springs to mind.
I do not know who invented this mixture but the sedative effects were far more pronounced although in it's favour, it did not seem to produce the aggression that some benzo cocktails cause. It was most certainly something you took as you sat down to watch a TV film.... so you could wake up as the credits went up the screen.
I did spend a bit of time looking for derivatives but it's low potency meant that it was not a useful target.
It's widely used within China and I do not think their is any legal control....
One Alexander R Surrey (Sterling Drug Company) spent a good decade of his career researching ths class and several
analogues proved to be more active but as usual, as soon as the marketing people found SOMETHING from which
to profit, they marketed it (and 1950s safety trials were not exactly great).
US3093639A
US3155655A
US3244703A
CN104817518A
I freely admit that I have not plowed through every related paper & patent but I am told that several homlogues are much like methaqualone. It's just the luck of the draw. One became a huge hit, the other is a footnote.
BTW I just remembered that the two enantiomers are said to possess quite different properties. The ADME of both are roughly the same but only one is an active anxiolytic. I believe that initially the designers intended to resolve the isomers BUT the only significant advantage was a faster onset and shorter duration - not big advantages for a medicine, possibly an advantage for an RC.




