haribo1
Ex-Bluelighter
- Joined
- Nov 29, 2006
- Messages
- 4,826
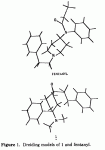
That super-potent mu agonist invented by Daniel Lednicer looks like this. All I can say is a)Wow, that's potent & b)it was never developed that far, so nobody has tried adding to, say, the cyclohexane ring (like 3-methyl fentanyl)

Has anyone come up with others working on these type of structures? I know the chinese replaced the phenylethyl group with a 2 thienyl ethyl group which was about 1/2 as potent. Beta -OH side-chain? WHo knows, but I suspect that this is the basis of a totally new class, rather than this 1 compound. At the very least, a 4-Fl group on the phenylethyl should help things a little. What is the radius of the bromine? Can pseudohalogens be used in place? Questions just keep popping up in my limited noggin...

Has anyone come up with others working on these type of structures? I know the chinese replaced the phenylethyl group with a 2 thienyl ethyl group which was about 1/2 as potent. Beta -OH side-chain? WHo knows, but I suspect that this is the basis of a totally new class, rather than this 1 compound. At the very least, a 4-Fl group on the phenylethyl should help things a little. What is the radius of the bromine? Can pseudohalogens be used in place? Questions just keep popping up in my limited noggin...