-
Neuroscience & Pharmacology Discussion Welcome Guest
Posting Rules Bluelight Rules Recent Journal Articles Chemistry Mega-Thread FREE Chemistry Databases! Self-Education Guide -
N&PD Moderators: Skorpio | someguyontheinternet
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
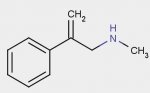
beta-methylene n-methyl phenethylamine
- Thread starter wungchow
- Start date
LuxEtVeritas
Bluelighter
nicer?
b-Me-PEA is of no notable effect as I am aware
b-Me-PEA is of no notable effect as I am aware
LuxEtVeritas
Bluelighter
i believe the b-Me barely has any MAOB metabolism inhibition over the parent (PEA)
MurphyClox
Bluelighter
- Joined
- Mar 26, 2008
- Messages
- 1,416
Back to this issue; I agree with L et V. So: About what kinda effects are we talking?LuxEtVeritas said:b-Me-PEA is of no notable effect as I am aware
fastandbulbous
Bluelight Crew
^ It's a decent decongestant & that's about it - has no CNS stimulant activity at all (tried it many years ago).
NeuronalPerception
Bluelighter
- Joined
- Mar 31, 2008
- Messages
- 216
I would stick with straight PEA.
LuxEtVeritas
Bluelighter
I;m sure you would 




Ham-milton
Bluelighter
- Joined
- Jul 20, 2007
- Messages
- 5,746
If only someone, somewhere could figure out where to stick a methyl on this molecule to make a decent recreational drug.
fastandbulbous
Bluelight Crew
^The alpha carbon, but that was thought of a fair while ago... 

Ham-milton
Bluelighter
- Joined
- Jul 20, 2007
- Messages
- 5,746
wow, way to ruin a joke.
MurphyClox
Bluelighter
- Joined
- Mar 26, 2008
- Messages
- 1,416
Uuuuh YEAH baby, that hits my taste...Ham-milton said:a decent recreational drug
Is there any report of CNS effect from any people from beta methyl?
I am just interesting in this as some people do get effect only from high dose PEA.
Anyway, if it is methylene, the conformation would change quite a lot (sp3 to sp2 making the angle between phenyl and alkyl change)
---------------------
Edit: Look like I was wrong, the methylene isnt that flat as I think, as i forgot about eclipsing hydrogen, in result it comes


A fast overlay and MM2 of (S)-isomer of betamethyl and the betamethyllene(displayed in yellow)
shows quite a bit similarity about angle

I am just interesting in this as some people do get effect only from high dose PEA.
Anyway, if it is methylene, the conformation would change quite a lot (sp3 to sp2 making the angle between phenyl and alkyl change)
---------------------
Edit: Look like I was wrong, the methylene isnt that flat as I think, as i forgot about eclipsing hydrogen, in result it comes


A fast overlay and MM2 of (S)-isomer of betamethyl and the betamethyllene(displayed in yellow)
shows quite a bit similarity about angle

Last edited:
fastandbulbous
Bluelight Crew
NeuronalPerception said:I would stick with straight PEA.
Why it's the same thing unless you eat a load of MAOI woith it, which isn't what's been asked (no ne of the PEA stuff you've mentioned in other threads without peer reviewed evidence of the evidence. I don't care hw many anecdotal things you say you have, it needs to be peer reviewed)
The related compound without the N-methyl group is part of a group of VMAT-2 (vesicular monamine transporter 2 ) inhibitors, the 3-amino-2-phenylpropenes. These should work to prevent VMAT-2 from taking cytosolic monoamines up into vesicles. They may even with appropriate substitution reverse VMAT-2. Both Methamphetamine and MDMA are known to alter VMAT activity and the ability of the vesicles to suck monoamines back into the vesicles. this combined with monoamine transport reversal pups monoamines out into the synapse.
I do not know whether they cause dumping of the vesicle contents by this mechanism alone rather than by the weak amine mechanism or some other mechanism as yet unknown
I was quite interested in these for a while, in particular the 3,4-methylenedioxyphenyl analog along with the potentially psychedelic 4-something 2,5-dimethoxy analogs I would expect that the methoxy substituted compounds would not retain VMAT activity whereas the 3,4-methylenedioxy would. If they do not have significant VMAT activity the potential psychedelic betamethylene compounds might have a future. this is pharmacological terra incognita and not somewhere I would want to go without animal tox testing.
The evidence as to the desirability of inhibiting VMAT and therefore increasing intracellular monoamine concentration is mixed. short term it probably does limited harm and it does seem to be part of the reason for the effects of MDMA/
the bad news:
1) Reserpine which is known to inhibit VMAT causing depletion of monamines in vesicles and is linked to severe depression, approximately 1 in 5 when it was widely used. Tetrabenazine another VMAT inhibitor is also linked to suicide and depression
2) The increased concentration of cytosolic monamines appears to be important in the neurotoxic effects of METH and MDMA.
3) Reduction in measurable VMAT is linked to depression and appears to play a part in the craving in cocaine addiction
for those interested:
the Bovine chromaffin transporter used in the study below is very similar to VMAT-2.
Perera R, Wimalasena DS, Kandatege W. Characterization of a series of 3-amino-2-phenylpropene derivatives as novel bovine chromaffin vesicular monoamine transporter inhibitors. J Med Chem. 2003;46: 2599-2605.
I do not know whether they cause dumping of the vesicle contents by this mechanism alone rather than by the weak amine mechanism or some other mechanism as yet unknown
I was quite interested in these for a while, in particular the 3,4-methylenedioxyphenyl analog along with the potentially psychedelic 4-something 2,5-dimethoxy analogs I would expect that the methoxy substituted compounds would not retain VMAT activity whereas the 3,4-methylenedioxy would. If they do not have significant VMAT activity the potential psychedelic betamethylene compounds might have a future. this is pharmacological terra incognita and not somewhere I would want to go without animal tox testing.
The evidence as to the desirability of inhibiting VMAT and therefore increasing intracellular monoamine concentration is mixed. short term it probably does limited harm and it does seem to be part of the reason for the effects of MDMA/
the bad news:
1) Reserpine which is known to inhibit VMAT causing depletion of monamines in vesicles and is linked to severe depression, approximately 1 in 5 when it was widely used. Tetrabenazine another VMAT inhibitor is also linked to suicide and depression
2) The increased concentration of cytosolic monamines appears to be important in the neurotoxic effects of METH and MDMA.
3) Reduction in measurable VMAT is linked to depression and appears to play a part in the craving in cocaine addiction
for those interested:
the Bovine chromaffin transporter used in the study below is very similar to VMAT-2.
Perera R, Wimalasena DS, Kandatege W. Characterization of a series of 3-amino-2-phenylpropene derivatives as novel bovine chromaffin vesicular monoamine transporter inhibitors. J Med Chem. 2003;46: 2599-2605.
fastandbulbous
Bluelight Crew
If they do not have significant VMAT activity the potential psychedelic betamethylene compounds might have a future. this is pharmacological terra incognita and not somewhere I would want to go without animal tox testing.
When I started whittering on about the SAR of 5HT2a agonists in 'Dragonflies, acid & the 5HT2a receptor', it was partly in the hope that someone might think it a reasonable suggestion & investigate their activity in a lab. Appears I'll just have to wait (possibly a bloody long time) to find out as my post grad days are long behind me now.
It'd be nice to find my hunch was right as I can talk shite for Britain sometimes!
the Betamethylene analog of 2-cb has been made and from what I understand assayed from very low doses up to the dose where if it was equipotent or more potent than 2-cb threshold psychedelic effects would have been seen, but they weren't.
these compounds are pretty accessible through the related acetophenone. But there is a complete lack of safety info even for distantly related compounds.
these compounds are pretty accessible through the related acetophenone. But there is a complete lack of safety info even for distantly related compounds.
fastandbulbous
Bluelight Crew
^ Oh well, just more fertilizer musing from me it seems 
I'm sure that they are active at some dose, just that they don't seem to be greater potency than their parent compounds. it would be interesting to see what shape the betamethylenes form in solution sort of real world conditions, rather than in silico modelling. COSY NMR anyone?