Giza
Ex-Bluelighter
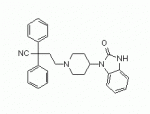
Aside from Etonitazene, Clonitazene, and Nitazene, is there any other Benzimidazoles or even analogs of the above worth investigation as supra potent opioid mu agonists? Un-specifically-scheduled... of course 
and does anyone know the mcg/kg approximate doses used for Etonitazene, Clonitazene and Nitazene?
and does anyone know the mcg/kg approximate doses used for Etonitazene, Clonitazene and Nitazene?