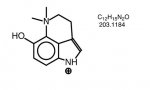
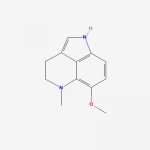
fastandbulbous
Bluelight Crew
^ Not only are they 'stench agents' (take the top off a bottle of 2-mercaptoethanol and tell me it smells nice, I dare you!), but they can form sulphide cross linkages with proteins of your body if you come across a denaturing agent & oxidizer.
Besides if you have an enzyme that's used to dealing with ethers and you give it a thioether to chew on, more often than not the thioether acts as a competetive inhibitor (the 2C-T-x/aleph compounds being a very good example) as it prevents the enzyme taking up the active conformation
Besides if you have an enzyme that's used to dealing with ethers and you give it a thioether to chew on, more often than not the thioether acts as a competetive inhibitor (the 2C-T-x/aleph compounds being a very good example) as it prevents the enzyme taking up the active conformation




 Never met a thiol I liked.
Never met a thiol I liked.